An hydroxytyrosol-based pharmaceutical formulation for the prevention of cardiovascular disease: a randomized controlled crossover trial.
Trombetta Domenico,1° Di Renzo Laura,2°* Smeriglio Antonella,1 Cascapera Stefano,3,4 Carmela Colica5
1 University of Messina, Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, Messina, Italy
2 Division of Clinical Nutrition and Nutrigenomic, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Rome, Italy
3 PhD School of Applied Medical-Surgical Sciences, University of Rome “Tor Vergata”, Rome, Italy
4 Iona Preparatory School, New Rochelle, NY
5 CNR, IBFM UOS of Germaneto, University “Magna Graecia” of Catanzaro, Campus “Salvatore Venuta”, Italy
° Equal contribution.
Introduction
In 2012 the 63% of worldwide deaths was been due to Non Communicable Diseases (NCDs), mainly cardiovascular disease (CVD), cancer, diabetes and chronic respiratory diseases. WHO projections expect a globally increase of 15% for NCDs deaths between 2010 and 2020.1 CVD represents the major cause of death in the world (29,6%), and in Europe, despite the recent slight decrease, the mortality for CVD is still consistent.1, 2
Among several CVD risk factors, obesity management and the reduction of bad eating habits could alleviate the increasing CVD gravity on worldwide deaths.
Inflammation contributes significantly to the development of chronic diseases including CVDs and cancer.3
Dietary supplementation with antioxidants, including phenolic compounds obtained from plants, may help maintain a desirable pro‑oxidative/antioxidative balance.4
Nowadays, different dietary sources are of considerable interest due to their potential health benefits in relation to different diseases.5
The efficacy of the Mediterranean diet, enriched with extra virgin olive oil (EVOO), was proved by the reduced incidence of major cardiovascular events in the Mediterranean population. This effect could be related to several changes of pathways involved in cardiometabolic risk, like resistance to oxidation and inflammation. In fact, the Mediterranean diet improves the non-enzymatic antioxidant capacity in order to protect the body from oxidative stress6,7 which plays a pivotal role in CVD onset and development.
EVOO supplementation brings several benefits on CVD, due to the antioxidant, anti-inflammatory, vasodilatory and antiplatelet aggregation proprieties of its phenolic compounds. The Food and Drug Administration (FDA) has recognized olive oil (23 g/day) as a qualified dressing able to decrease the risk of coronary heart disease.8
The main EVOO cardio-protective effect is attributable to the presence of its phenolic compounds.9 Indeed, the hydroxytirosol (2–(3,4-dihydroxyphenyl) ethanol) (HT) has good effects on the prevention of CVD, High-Density Lipoprotein cholesterol (HDL-C) and antioxidant enzymes activity.10,11
Atherosclerosis is characterized by a strong imbalance between oxidants and antioxidants, responsible for CVD onset in subjects with a high-grade inflammation. HT plays a significant role in CVDs prevention, and its metabolites are able to protect from endothelial dysfunction commonly present in atherosclerosis.12 HT is able to reduce cardiomyocyte apoptosis, infarct size, hypercholesterolemia levels, inhibit peroxidation of Low Density Lipoprotein cholesterol (LDL-C), reduce markers of lipid peroxidation like malondialdehyde (MDA), and increase HDL-C.13,14
In vitro and in vivo studies15 showed an increased expression of Superoxide dismutase-1 (SOD1) after EVOO and HT acute intake, demonstrating the ability of olive oil phenolic compounds to improve the endogenous antioxidant defense.
SOD1 is a cytoplasmic antioxidant enzyme that plays a crucial role in hypertension and atherosclerosis onset.16 SOD1 is able to catalyze superoxide radical (O2·-) in hydrogen peroxide (H2O2), which in turn is catalyzed into water through glutathione peroxidase (GSH-Px) and catalase (CAT).16,17 Superoxide, controlled by SOD1, belongs to the Reactive Oxygen Species (ROS), and is involved in several endothelial dysfunctions. Therefore, we know that superoxide plays an important role in the development of atherosclerosis. In addition to SOD1, GSH-Px and CAT, other enzymes are involved in the regulation of oxidative stress. These includes the macrophage migration inhibitory factor (MIF)18 and peroxisome proliferator-activated receptor gamma (PPARγ).19 Conversely, ROS have the ability to modulate the expression of different inflammation genes like nuclear factor of kappa light polypeptide gene enhancer in B-cells (NFKB),20 and consequently chemokine (C-C Motif) ligand 2 (CCL2).21
To our knowledge, no research has tested the acute effects of a new pharmaceutical formulation containing EVOO enriched with HT on biomarkers associated with CVD (glycemic and lipid profiles) and antioxidant status. Moreover, the literature is lacking studies that evaluate the aspects of nutrigenomics including gene expression modulation of oxidative stress, inflammatory pathway, risk of sarcopenia and osteoporosis.
Therefore, the purpose of our study was to assess, through a nutrigenomic approach, the antioxidant effects of HT and how they could exert a lipoperoxyl radical-scavenging activity, able to modulate gene expression of genes related to inflammatory pathways, and increase the levels of antioxidant compounds.
Our hypothesis is that the acute treatment with hydroxytyrosol (HTT) may help reduce the oxidative stress in postprandial time. Hence, we wanted to test the efficacy in healthy human volunteers of 15 mg/day of HT, extracted from olive fruits and conveyed in organic EVOO, present in a new formulation with intestinal release, on glycemic and lipid profile, oxidative stress biomarkers and genomics. A randomized controlled trial was performed on healthy volunteers.
Methods
Study design and subjects
The study was conducted using a randomized, controlled crossover design, between December 2015 and April 2016. Subjects were recruited sequentially within a routine medical check-up program at the Section of Clinical Nutrition and Nutrigenomics, Department of Biomedicine and Prevention of the University of Rome “Tor Vergata”.
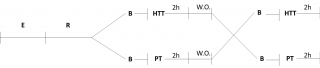
The study consists of HTT or placebo (PT), separated by a 2-weeks wash out period. At the end of wash out, the groups received PT or HTT (Figure 1).
Figure 1. Clinical study design.
The European Food Safety Authority (EFSA) established for the EVOO polyphenol the health claim “protection of blood lipids from oxidative stress”. This health claim is valid only if the daily intake of HT and its derivatives is at least 5mg/day, in order to have a protection of LDL particles from oxidative damage.22 In the present study, the administration of 15 mg/day of HT was established in order to guarantee the achievement of recommended dose.
Eligible patients were randomly assigned to HTT and PT in a 1:1 ratio.
Randomization was performed by a person from the clinical investigation unit not involved in the clinical trial. The study was therefore double-blind.
Volunteers took daily n. 2 capsules of 7.5 mg HT or n. 2 capsules of placebo (P) administered in randomized order, before lunch in order to ensure gastric transit and intestinal absorption.
Subjects were asked to maintain their usual lifestyle habits and to report any illness or abnormality arising during the study. Throughout the study, volunteers were restricted regarding their consumption of polyphenol-rich drinks like coffee, tea, wine and cocoa (less than 200 mL/day).
Nutritional status and body composition were evaluated with anthropometry, dual X-ray Absorptiometry (DXA) and bioimpedentiometry (BIA), and blood analysis. The evaluation was performed at baseline (B) to identify eligible subjects.
At baseline and at post prandial time, after two hours, gene expression and blood analysis were performed.
Due to the different phenotypes of obesity, according to De Lorenzo et al.,23 subjects were classified based on body mass index (BMI) and percentage (%) of total body fat (TBFat) as: normal weight lean subject with BMI <25kg/m2 and TBFat (%)<30; normal weight obese subject with BMI <25 kg/m2 and TBFat (%) ≥30; pre obese/obese subject with BMI ≥25 kg/m2 and TBFat (%) ≥30.
All participants recruited in the study authorized their participation by reading and signing the informed consent, conducted in accordance with the Helsinki Declaration of 1975 as revised in 1983.
The study has been registered in ClinicalTrials.gov Id: NCT01890070 (www.ClinicalTrials.gov).
Endpoint
The primary endpoint of this study was to asses a panel of blood analys to define glycemic and lipid profiles (insulin; glucose; triglycerides, Tg; total cholesterol, TC; HDL-C; LDL-C), antioxidant status (Total Antioxidant Status (TAS) and lipid peroxidation, expressed as MDA, Thiols, Nitrites/Nitrates), plasma and erythrocytes membrane polyunsatured fatty acids (PUFAs).
The secondary endpoint was to evaluate HTT on gene expression of selected genes belonging to inflammatory and oxidative stress pathway: MIF, SOD1, PPARγ, CAT, CCL2, NFkB1, methylenetetrahydrofolate reductase (MTHFR), apolipoprotein E (APOE), angiotensin I converting enzyme (ACE), vitamin D (1,25-dihydroxyvitamin D3) receptor (VDR), and interleukin-6 receptor (IL-6R).
Inclusion and exclusion criteria
Inclusion criteria: healthy volunteers, according to blood analysis, aged between 18 and 65 years.
Exclusion criteria: pregnancy; breast-feeding; type 1 and type 2 diabetes; heart failure; endocrine disorders; liver dysfunction; liver, kidney, autoimmune, chronic viral (Hepatitis C, B) and neoplastic diseases; corticosteroid and chronic inflammatory therapy; participation in other dietary trials.
Sample size
As insulin was a parameter of the first outcome, it was selected to calculate minimum sample size according to de Bock M. et al.24 The minimum sample size was calculated using a two-tailed one-sample Student’s t-test, considering: (i) insuline to be detected between baseline and HTT |δ| ≥15 µU/mL – 1; (ii) SD of the paired differences SD = 15 µU/mL; (iii) type I error probability α = 0.05 and power 1 − β = 0.90. The result was a minimum sample size of 10 per group.
HT and placebo capsules composition
HT capsules were composed of: Hard Gelatin Enteric Coated Capsules (Fenolia™, P&P Farma Srl, Turin, Italy) at 7.5 mg strength of Hydroxytyrosol from Olive Extract (elaVida™, DSM, Heerlen, The Netherlands) in Extra Virgin Organic Olive Oil vehicle and enteric coated with Eudraguard® natural (Evonik Industries AG, Essen, Germany). Placebo capsules were composed of: gelatin; coating agent: modified starch; anti-caking agents: talc, silicon dioxide; stabilizer: glycerol; dl-alpha-tocopherol; dyes: E171, E141, E161b.
Anthropometric evaluation
Waist and hip circumferences were taken using a flexible steel metric tape to the nearest 0.5 cm, with subjects standing with arms relaxed by their side and balanced on both feet. Waist circumference was measured just above the iliac crest. Hip circumference measurement was taken at the greatest posterior protuberance of the buttocks. Waist/hip ratio (WHR) was also evaluated in relation to clinical risk thresholds, i.e. WHR>1 for men and WHR>0.9 for women. Body weight (kg) was measured to the nearest 0.1 kg, using a technical balance (Invernizzi, Rome, Italy). Height (m) was measured to the nearest 0.1 cm using a stadiometer (Invernizzi, Rome, Italy).
Body mass Index (BMI) was calculated using the formula: BMI = body weight /height2 (kg/m2).
Body composition analysis
Dual X-ray Absorptiometry (DXA) and plicometry
TBfat, lean mass (TBLean) and total body bone mass (TBBone) were assessed using dual-energy X-ray absorptiometry (DXA) (i-DXA, GE Medical Systems, Milwaukee, WI, USA), following the previously described procedure.23 The average measurement time was 20 min. The effective radiation dose from this procedure is about 0.01 mSv. The coefficient of variation (coefficient of variation = 100 x SD/mean) intra and inter subjects ranged from 1% to 5%. The coefficient of variation for bone measurements is less than 1%; coefficient of variation on this instrument for five subjects scanned six times over a nine month period were 2.2% for TBfat, and 1.1% for TBLean.
TBfat % = (TBfat+ TBLean + TBBone) x 100.
TBfat distribution was also determined using a plicometer (Harpenden Callipers) with skinfolds measurement in triplicate and collected at four points: triceps, biceps, subscapular and suprailiac. Skinfolds measurements were utilized in Durnin & Rahman (1974) equations to calculate body density, and the Siri formula was subsequently applied to predict TBfat %.25
Bioimpedentiometry
Resistance, reactance, impedance and phase angle at 50 kHz frequency were measured using a BIA phase sensitive system (BIA 101S, Akern/RJL Systems, Florence, Italy). Total body water (TBW), extracellular water (ECW), intracellular water (ICW), Na/K ratio, phase angle (PA), body cell mass (BCM), and body cell mass index (BCMI) were calculated from bioelectrical measurements and anthropometric data employing the software provided by the manufacturer, which incorporates validated predictive equations.23
Analysis of Blood Samples
Biochemical analysis
Blood samples were taken after a 12-hour overnight fast. Blood samples were collected in sterile tubes containing EDTA (Vacutainer®) and placed on ice. Plasma was separated by centrifugation (1600 rpm, at 4°C for 10 min), removed, aliquoted and stored at -80°C. All clinical chemistry analyses, except plasma glucose and serum lipid analysis, were carried out using an ADVIA®1800 Chemistry System (Siemens Healthcare) following standard procedures.26 Plasma glucose concentrations were measured using the glucose oxidase method and automated glucose analyzer (COBAS INTEGRA 400, Roche Diagnostics, Indianapolis, IN, USA); serum lipid profile components were determined by standard enzymatic colorimetric techniques (Roche143 Modular P800, Roche Diagnostics, Indianapolis, IN, USA). Cardiovascular risk was determined by the following ratios: TC/HDL-C (<3); LDL-C/HDL-C (<2).
Adipocyte dysfunction was evaluated by the lipid accumulation product index (LAP) calculated using the following formulas:
(Waist circumference [cm] – 65) x Tg concentration [mM] for men;
(Waist circumference [cm] – 58) x Tg concentration [mM] for women.
All the analyses were performed in duplicate, while the measurements were performed in triplicate, at Polyclinic of Tor Vergata, Rome, Italy.
Antioxidant status
TAS, MDA, Thiols, Nitrites/Nitrates were determined using commercially available kits following manufacturer’s instructions. Fluorometric Thiol Assay Kit (MAK151-1KT), MDA Assay Kit (MAK085-1KT) and Nitrite/Nitrate Assay Kit (23479-1KT-F) were purchased from Sigma-Aldrich (Milan, Italy). Total Antioxidant Status Assay Kit (615700-1KIT) was purchased from Merck Millipore (Milan, Italy). Oxidized LDL-C (ox-LDL) concentration in plasma was measured by enzyme linked immunosorbent assay using the mAb-4E6 antibody (Mercodia AB, Uppsala, Sweden).
PUFAs were analyzed from venous blood sample and collected in tubes containing EDTA (Vacutainer®) and centrifuged (3500 rpm for 5 min). Plasma was removed and stored at -80°C while red cells were washed with saline solution (NaCl 0,9%) and collected and frozen at - 80°C. Erythrocyte membrane PUFAs methyl esters were extracted by a modified version of previously reported methods.27 In plasma, total PUFAs methyl esters were evaluated through gas chromatography (GC, model 6890N, Agilent Technologies Italia Spa), coupled to a mass spectrometric detector (MSD, model Agilent 5973 inert, Agilent Technologies Italia Spa). Chromatographic separation was performed on a BPX-70 (70% cyanopropyl polysilphenylensiloxane, SGE Analytical Science, Austin, TX, USA) 30m capillary column (0.25mm ID, 0.25μm film thickness).
All the analyses were performed in triplicate.
Antioxidant status was performed at Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Italy.
Sample collection, RNA extraction and analysis
Blood samples were collected, stabilized in PAX gene Blood RNA Tubes (Pre AnalytiX Qiagen, Hombrechtikon, Switzerland) and stored at -80°C. Total RNA of each collected sample was purified with PAX gene Blood miRNA Kit following the manufacturer’s instructions (Pre Analytix Qiagen, Hombrechtikon, Switzerland). Total RNA was quantified, assessed for quality by spectrophotometry (Nanodrop, Wilmington, USA) and agarose gel electrophoresis. Specific RT2 Profiler PCR Arrays (Qiagen, Netherlands) were used, focusing on the Human Oxidative Stress (PAHS-065ZA, Qiagen, Netherlands) and Human Inflammation (PAHS-097ZA, Qiagen, Netherlands) pathways. Gene expression of 13 genes was analyzed.
Specific RT2 Profiler PCR Arrays (PAHS-065ZA, Qiagen, Netherlands) were analyzed for human oxidative stress: SOD1 (GenBank accession n. AK312116.1), and CAT (GenBank accession n. AY545477.1).
Specific RT2 Profiler PCR Arrays (PAHS-097ZA, Qiagen, Netherlands) were analyzed for human inflammation pathways: MIF (GenBank accession n. AF469046.1), PPARγ (GenBank accession n. AB307692.1), CCL2 (GenBank accession n. AK311960.1), NFkB1 (GenBank accession n. AK1122850.1), IL6R.
Gene expression of cardiovascular risk related genes was analyzed: VDR, MTHFR (GenBank accession n. AB209113.1), APOE (G enBank accession n. K314898.1) and ACE (GenBank accession n. AB208971.1). Each qRT-PCR experiment was performed in triplicate and repeated at least twice, in line with manufacturer’s instructions (Qiagen, Netherlands).
Genomic analyses were performed at the Division of Clinical Nutrition and Nutrigenomics, University of Rome Tor Vergata, Italy.
HT bioavailability determination
The HPLC method for HT bioavailability determination was validated in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use; Harmonized Tripartite Guideline (2005),28 in terms of selectivity, linearity, limit of detection and quantitation and precision. A good analytical method should be able to measure accurately the analyte in the presence of suspected interferences such as its own degradation products and any co-eluting compounds. The chromatographic separation of HT showed no overlap (base-line separations); also, as highlighted by chromatogram, no interferences by matrix constituents (EVOO polyphenols) and excipients during the analyte retention time were found (Figure 2).
Figure 2. Exemplificative HPLC chromatogram of qualitative-quantitative analysis of HT in plasma sample.
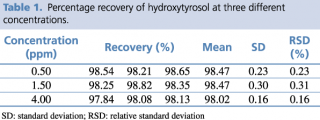
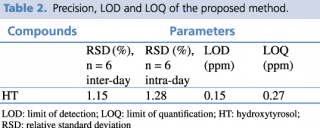
A test solution containing different concentrations of pure reference standard was prepared and analyzed using the analytical parameters described earlier. The detection limit was calculated as the amount of chemical that resulted in a peak three times higher with respect to baseline noise. The linear calibration range, regression equation, quantification limit and precision, expressed as relative standard deviation percentage (RSD%) of the compound of interest, were calculated, and the results are presented in Tables 1 and 2.
Table 1. Percentage recovery of hydroxytyrosol at three different concentrations.
Table 2. Precision, LOD and LOQ of the proposed method.
HT was extracted from acidified plasma by solid-phase extraction using an Oasis HLB 1 cc Vacuum Cartridge 30 mg, 30 µm (Waters, Italy) following the method developed by Ruiz-Gutierrez and coworkers.29
The cartridge was placed in a vacuum system device fitted with a small vacuum pump. Pressure was monitored during the critical cartridge conditioning, equilibration, and sample loading steps, thereby preventing fluctuations in separation efficiency due to variations in flow rate. Prior to use, the cartridge was conditioned with 1 mL of methanol followed by equilibration using 1 mL of H2O. The sample solution, consisting of 100 µL of plasma sample, 10 µL of H3PO4 8.5% and 10 µL of gallic acid 1 mg mL-1 as internal standard, was loaded. The cartridge was washed with 0.5 mL of water followed by 1 mL of 5% (v/v) methanol in water and then the sample was eluted with 1.5 mL of methanol. The eluted fraction was filtered through a 0.22 µm nylon membrane filter and injected into an HPLC system.
A 500 µg mL−1 HT standard stock solution was prepared using acetic acid 0.2% and methanol 75/25 (v/v) (pH 3.30). From this solution, freshly working standard solutions from 3 to 100 µg mL−1 were prepared. HT (2-(3,4-di-hydroxyphenyl) ethanol) and gallic acid ≥ 98% were purchased from Sigma-Aldrich (Milan, Italy). Glacial acetic acid, phosphoric acid and methanol were HPLC grade and purchased from Merck (Darmstadt, Germany). Water used in all experiments was passed through a Milli-Q (Millipore, Milan, Italy) water purification system (18 mΩ).
The analytical evaluation was performed following Miralles et al.30 with some modifications using an Agilent high performance liquid chromatography system (1100 series) equipped with an LC binary pump, an autosampler, a degasser and a thermostatic column oven coupled to a UV-Vis photo diode array detector (DAD). The column was an Ascentis 150 x 4.6 mm, 5µm (Supelco, Milan, Italy). The elution gradient consisted of mobile phase (A) H2O (0.2% CH3COOH, pH 3.1) and (B) CH3OH. The following gradient was used: 0-2 min, 95% A and 5% B; 10 min, 75% A and 25% B; 13 min, 95% A and 5% B; 13-15 min 95% A and 5% B. Flow rate was 1.5 mL/min and injection volume was 20 µL. The column oven was set at 25°C. HT was recognized and quantified at 280 nm based on the supplier’s standard, comparing retention time and relative UV-VIS spectra (range 200-400 nm). Results are expressed as µg of HT per ml of plasma sample.
All the analyses were performed in triplicate.
HT bioavailability determination was performed at Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Italy.
Statistical analysis
Statistical analysis was carried out using IBM SPSS 21.0 for Windows (Armonk, NY: IBM Corp. USA). A descriptive statistical analysis was conducted for body composition and blood parameters.
After the Shapiro-Wilk test, a paired t test or a non-parametric Wilcoxon test was performed to evaluate differences before and after treatment (HTT and PT). In all statistical tests performed, the null hypothesis (no effect) was rejected at the 0.05 level of probability.
Data Analysis of Quantitative Real Time PCR: the value used to plot relative gene expression was determined using the expression Fold Change (FC)=2-ΔΔCT,31 using β-actin (ACTB) as housekeeping gene. Lastly, only genes with a FC>2 were selected and referred to as “differentially expressed genes”, with a p value <0.05 taken to indicate statistical significance.
Results
Of the forty enrolled subjects, twelve of them were excluded from the trial (four subjects didn’t meet inclusion criteria, while eight subjects declined to participate). Finally, twenty-eight patients completed the trial.
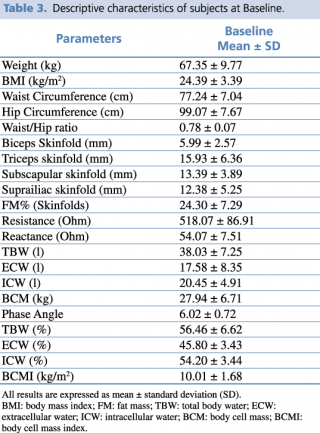
No changes to trial outcomes after the trial commenced occurred. The descriptive characteristics of the enrolled subjects are indicated in Table 3.
Table 3. Descriptive characteristics of subjects at Baseline.
All subjects were in good health conditions. The average age of subjects was 32.00 ± 12.22 years, 57% female and 43% male.
Subjects’ phenotypes were as follows: 42.8% Normal Weight Lean, 28.6% Normal Weight Obese and 26.8% Pre-obese/Obese. The mean ± SD of FM% was 28.26 ± 10.43, and of FM (kg) 16.10 ± 6.91.
Median value of HT bioavailability was of 0.25 mg/ml of plasma.
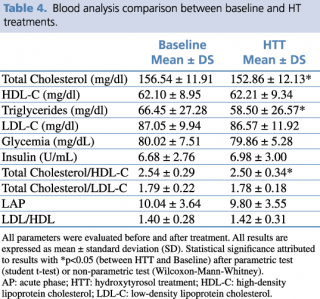
HTT showed significant reductions in levels of total cholesterol (p=0.026) and triglycerides (p= 0.011), (Table 4).
Table 4. Blood analysis comparison between baseline and HT treatments.
Consequently, cardiovascular risk index, evaluated by total cholesterol/HDL, was significantly reduced (p= 0.008). We also detected a significant increase in LDL cholesterol levels (p= 0.026; Δ%= 6.32%), albeit remaining within normal range.
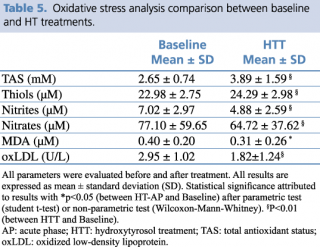
The results obtained show a marked antioxidant effect on all assessed parameters. In fact, thiol groups and TAS were already significantly higher than baseline (respectively, p= 0.004, and p= 0.002). By contrast, lipid peroxidation showed a marked and statistically significant reduction with respect to baseline values of nitrite and nitrate (p= 0.001, and p= 0.001), oxLDL (p<0.001), and MDA (p= 0.019) (Table 5).
Table 5. Oxidative stress analysis comparison between baseline and HT treatments.
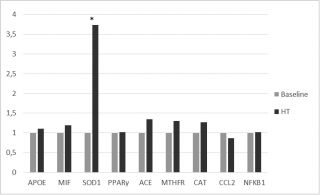
A moderate up-regulation of MTHFR and ACE gene expression was observed. Moreover, significant up-regulation of SOD 1, with a fold-change exceeding the threshold set at 2, was observed (2-ΔΔCt = 6.2) (Figure 3).
Figure 3. Gene expression Fold Change after HT treatments.
No significant changes after PT were observed.
Discussion
EVOO is the main lipid component of the Mediterranean Diet.32 EVOO contains phenolic compounds, as HT, that have several beneficial effects on lipoproteins, inflammatory markers, oxidative damage, cellular and platelet functions, bone integrity and antimicrobial activity.33,34
Even if HT is easily absorbed in the digestive tract, its bioavailability is limited by the production of sulphate and glucuronide conjugate during their first metabolic pass in gut and liver. The absorption and the exerted effects of phenolic compounds depend on their dosage. Compared to intravenous administration, the ingestion of HT is more effective in terms of bioavailability.35
Unfortunately, literature presents only few data, with limited knowledge of HT absorption in humans. Pharmacokinetic studies were performed in order to understand the bioavailability of HT in olive oil.36 The substantial differences observed in the absorption of HT, when administered in EVOO from its pure form as aqueous solution, can be explained by the presence of other polyphenols naturally present in olive oil, such as oleuropein and other secoiridoids, which can release HT by hydrolysis in the intestine.33
Moreover, since the olive oil polyphenols are mainly absorbed in the small intestine and in the colon,37 the gastric emptying time and HT slow release from EVOO matrix determine extended absorption curves38 responsible for the high HT concentrations in plasma after chronic nutritional intervention.
For the first time, we tested the bioavailability and the effects of a new hydroxytyrosol-based pharmaceutical formulation in a randomized controlled crossover trial. In our study, all genomic and metabolomics results reflect the high grade of bioavailability of HT supplements. The presence of 0.25 mgml-1 of HT in plasma proved that the particular gastro resistant formulation allows the HT, as well as other EVOO polyphenols, to be released in the intestine without suffering degradation in the stomach. Our results confirmed literature data on the better absorption at the intestinal level of HT by EVOO.39,40 Furthermore, the presence of other antioxidant agents naturally present in EVOO added in the new formulation that we tested, such as α-tocopherol, could prevent the metabolism of phenols and help increase the bioavailability of HT compared to other commercially available formulations.
Significant effect of 15 mg/day of HT intake compared to placebo, on appropriate markers of oxidation, has been highlighted by this study. TAS is a reflection of oxidative stress, and growing of TAS concentration after treatment indicates that HT supplementation leads to the improvement of oxidant activity. Moreover, the increased thiols concentration could implicate a prominent role of the phenolic compound in the maintenance of redox balance. This phenomenon is probably due to its antioxidant activity, which protects endogenous antioxidant molecules from oxidation.
High LDL-C and total cholesterol levels are considered as risk factors for cardiovascular disease (CVD). On the other hand, high HDL levels have a protective role for CVD. HDL-C plasma concentration increases with high consumption of phenolic compounds, which contribute to lowering LDL-C levels.10,11,36
As suggested by literature,41-43 in our study HTT is able to reduce hypercholesterolemia, nitrite, nitrate, lipids peroxidation and ox-LDL-C concentrations.44 Other studies, also, demonstrated that the consumption of high doses of phenolic compounds reduced the risk factors linked to the development of coronary heart disease. Hence, we observed significant decreases of -1.88% of total cholesterol level and -9.40% of triglycerides, combined with a significantly reduction of cardiovascular risk index (Δ%= -3.65 %), after HTT respect to placebo.
In accordance to Katsarou et al.11 we demonstrated that HTT is able to reduce lipids peroxidation and protect cells membrane and other molecules from oxidation and prevent the atherosclerosis risk.
Blood analysis and gene expression were going in the same direction.
Our transcriptome study also revealed that the majority of studied genes induced lower fold-changes. Nevertheless, the up regulation of SOD1, a cytoplasmic enzyme involved in the pathogenesis of various diseases among which atherosclerosis onset and hypertension,16,17 was significantly increased after HTT.
Increasing expression of SOD1 could be related to critical reduction of nitrite and nitrate concentrations, and this hypothesis is supported by Yao Q. et al. results.45
This finding represents the first evidence of a role for HT in the gene-mediated effects related to the reduction of biomarkers of oxidative stress.
However, no significant effects of HTT were observed in the regulation of inflammation mediated by NFKB.
Furthermore, our results are sufficient to suggest that a daily intake of 15 mg of HT could be recommended to decrease oxidative stress and prevent cardiovascular risk, due to lipid and plasma antioxidant profile improvement.
A limit of the study was the lack of certain enzymes activity measurement, such as SOD and CAT that could be related to antioxidant effects of HT.
Other limits of this study were the small number of enrolled subjects and short duration of treatment. However, the calculated sample size has been chosen regarding previous nutrigenomic studies in clinical trials,46 and on the basis of minimal sample size.
The idea that it is possible to reduce the postprandial oxidative stress that leads to a basis for acute cardiovascular damage may seem trivial. Although our results do not allow to conclude that an acute intake of HT can prevent CVD, it seems reasonable to assume that a favorable acute effect is shown on some risk factors, such as oxidative stress biomarkers, over expression of inflammation and oxidative stress related genes. We know that continuous oxidative insults can increase the risk of CVD. Therefore, the possibility of reducing the oxidative processes with acute treatments could prevent this risk, day by day.
Moreover, according to our results, n.2 capsules/day containing 7.5 mg of HT could be proposed to EFSA opinion to obtain a “health claim” according to Regulation (CE) n. 1924/2006, related to the following nutritional and health indications: “reduces oxidative stress”, “maintains normal blood HDL-cholesterol concentrations”, has “anti-inflammatory properties”, “antioxidant properties”, and “contributes to body defenses against external agents”.
Further clinical trials with this new HT formulation, on a larger population, over a longer period, to increase knowledge about therapeutic mechanisms and to ensure its efficacy and safety, before definitive conclusions can be made, are required.
Acknowledgments:
We are indebted to all the subjects who volunteered in the clinical trial. We also thank Doctor Paola Gualtieri for statistical analysis of data, Doctor Giorgia Cioccoloni for technical research assistance and the entire medical team from the clinical research unit for their technical assistance in conducting the clinical aspects of this study. We are indebted to Doctor Gemma Lou De Santis for English language revision.
Supported by grants from Ministry of Agriculture, Food and Forestry (D.M.: 2017188 03/24/2011).
Author Contributions: TD contributed to the interpretation of the data and drafted the manuscript; DRL conceived, designed the experiments, and drafted the manuscript; SA, CS collected the data and performed the experiments; CC had primary responsibility for the final content. All the authors read and approved the final manuscript. All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of Interest. No conflicts of interest, financial or otherwise are declared by the authors.
References
- Organization WH. Global Status Report on Noncommunicable Diseases 20142014. 298 p.
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular Disease in Europe 2014: Epidemiological Update. European Heart Journal. 2014;35(42):2950-9.
- Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135-43.
- Halliwell B, Rafter J, Jenner A. Health Promotion by Flavonoids, Tocopherols, Tocotrienols, and Other Phenols: Direct or Indirect Effects? Antioxidant or Not? The American Journal of Clinical Nutrition. 2005;81(1 Suppl):268s-76s.
- Calder PC. The Role of Marine Omega-3 (N-3) Fatty Acids in Inflammatory Processes, Atherosclerosis and Plaque Stability. Molecular Nutrition & Food Research. 2012;56(7):1073-80.
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. The New England Journal of Medicine. 2013;368(14):1279-90.
- Zamora-Ros R, Serafini M, Estruch R, Lamuela-Raventos RM, Martinez-Gonzalez MA, Salas-Salvado J, et al. Mediterranean Diet and Non Enzymatic Antioxidant Capacity in the PREDIMED Study: Evidence for a Mechanism of Antioxidant Tuning. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD. 2013;23(12):1167-74.
- de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications after Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779-85.
- Covas MI, Nyyssonen K, Poulsen HE, Kaikkonen J, Zunft HJ, Kiesewetter H, et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized Trial. Annals of Internal Medicine. 2006;145(5):333-41.
- Bulotta S, Celano M, Lepore SM, Montalcini T, Pujia A, Russo D. Beneficial Effects of the Olive Oil Phenolic Components Oleuropein and Hydroxytyrosol: Focus on Protection Against Cardiovascular and Metabolic Diseases. Journal of Translational Medicine. 2014;12:219
- Katsarou AI, Kaliora AC, Chiou A, Kalogeropoulos N, Papalois A, Agrogiannis G, et al. Amelioration of Oxidative and Inflammatory Status in Hearts of Cholesterol-Fed Rats Supplemented with Oils or Oil-Products with Extra Virgin Olive Oil Components. European Journal of Nutrition. 2016;55(3):1283-96.
- Meza-Miranda ER, Rangel-Zuniga OA, Marin C, Perez-Martinez P, Delgado-Lista J, Haro C, et al. Virgin Olive Oil Rich in Phenolic Compounds Modulates the Expression of Atherosclerosis-Related Genes in Vascular Endothelium. European Journal of Nutrition. 2016;55(2):519-27.
- Catalan U, Lopez de Las Hazas MC, Rubio L, Fernandez-Castillejo S, Pedret A, de la Torre R, et al. Protective Effect of Hydroxytyrosol and Its Predominant Plasmatic Human Metabolites against Endothelial Dysfunction in Human Aortic Endothelial Cells. Molecular Nutrition & Food Research. 2015;59(12):2523-36.
- Mitjavila MT, Moreno JJ. The Effects of Polyphenols on Oxidative Stress and the Arachidonic Acid Cascade. Implications for the Prevention/Treatment of High Prevalence Diseases. Biochemical Pharmacology. 2012;84(9):1113-22.
- Ilavarasi K, Kiruthiga PV, Pandian SK, Devi KP. Hydroxytyrosol, the Phenolic Compound of Olive Oil Protects Human PBMC against Oxidative Stress and DNA Damage Mediated by 2,3,7,8-TCDD. Chemosphere. 2011;84(7):888-93.
- Fukai T, Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxidants & Redox Signaling. 2011;15(6):1583-606.
- Gregory EM, Fridovich I. Oxygen Toxicity and the Superoxide Dismutase. Journal of Bacteriology. 1973;114(3):1193-7.
- Koga K, Kenessey A, Ojamaa K. Macrophage Migration Inhibitory Factor Antagonizes Pressure Overload-Induced Cardiac Hypertrophy. American Journal of Physiology Heart and Circulatory Physiology. 2013;304(2):H282-93.
- Fischer B, von Knethen A, Brune B. Dualism of Oxidized Lipoproteins in Provoking and Attenuating the Oxidative Burst in Macrophages: Role of Peroxisome Proliferator-Activated Receptor-Gamma. Journal of Immunology (Baltimore, Md : 1950). 2002;168(6):2828-34.
- Gloire G, Legrand-Poels S, Piette J. NF-Kappab Activation by Reactive Oxygen Species: Fifteen Years Later. Biochemical Pharmacology. 2006;72(11):1493-505.
- Chen YM, Chiang WC, Lin SL, Wu KD, Tsai TJ, Hsieh BS. Dual Regulation Of Tumor Necrosis Factor-Alpha-Induced CCL2/Monocyte Chemoattractant Protein-1 Expression in Vascular Smooth Muscle Cells by Nuclear Factor-Kappab and Activator Protein-1: Modulation by Type III Phosphodiesterase Inhibition. The Journal of Pharmacology and Experimental Therapeutics. 2004;309(3):978-86.
- Efsa Panel on Dietetic Products N, Allergies. Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance Of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2011;9(4):2033-N/A.
- De Lorenzo A, Bianchi A, Maroni P, Iannarelli A, Di Daniele N, Iacopino L, et al. Adiposity Rather than BMI Determines Metabolic Risk. International Journal of Cardiology. 2013;166(1):111-7.
- de Bock M, Derraik JG, Brennan CM, Biggs JB, Morgan PE, Hodgkinson SC, et al. Olive (Olea Europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. Plos One. 2013;8(3):E57622.
- Durnin JV, Womersley J. Body Fat Assessed from Total Body Density and Its Estimation from Skinfold Thickness: Measurements on 481 Men and Women Aged from 16 to 72 Years. The British Journal of Nutrition. 1974;32(1):77-97.
- Graesli AR, Fahlman A, Evans AL, Bertelsen MF, Arnemo JM, Nielsen SS. Haematological and Biochemical Reference Intervals for Free-Ranging Brown Bears (Ursus Arctos) in Sweden. BMC Veterinary Research. 2014;10:183.
- Bligh EG, Dyer WJ. A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911-7.
- (ICH) Icoh. Technical Requirements for Registration of Pharmaceuticals for Human Use. Topic Q2 (R1): Validation of Analytical Procedures: Text and Methodology. Geneva, Switzerland 2005 [Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- Ruiz-Gutierrez V, Juan ME, Cert A, Planas JM. Determination of Hydroxytyrosol in Plasma by HPLC. Analytical Chemistry. 2000;72(18):4458-61.
- Miralles P, Chisvert A, Salvador A. Determination of Hydroxytyrosol and Tyrosol by Liquid Chromatography for the Quality Control of Cosmetic Products Based on Olive Extracts. Journal of Pharmaceutical and Biomedical Analysis. 2015;102:157-61.
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and The 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402-8.
- Alberti-Fidanza A, Fidanza F, Chiuchiu MP, Verducci G, Fruttini D. Dietary Studies on Two Rural Italian Population Groups of the Seven Countries Study. 3. Trend of Food and Nutrient Intake from 1960 to 1991. European Journal of Clinical Nutrition. 1999;53(11):854-60.
- Visioli F, Galli C, Bornet F, Mattei A, Patelli R, Galli G, Et Al. Olive Oil Phenolics Are Dose-Dependently Absorbed in Humans. FEBS Letters. 2000;468(2-3):159-60.
- Weinbrenner T, Fito M, De La Torre R, Saez GT, Rijken P, Tormos C, Et Al. Olive Oils High in Phenolic Compounds Modulate Oxidative/Antioxidative Status in Men. The Journal of Nutrition. 2004;134(9):2314-21.
- Tuck KL, Freeman MP, Hayball PJ, Stretch GL, Stupans I. The In Vivo Fate of Hydroxytyrosol and Tyrosol, Antioxidant Phenolic Constituents of Olive Oil, after Intravenous and Oral Dosing of Labeled Compounds to Rats. The Journal of Nutrition. 2001;131(7):1993-6.
- Covas MI, de la Torre K, Farre-Albaladejo M, Kaikkonen J, Fito M, Lopez-Sabater C, et al. Postprandial LDL Phenolic Content and LDL Oxidation Are Modulated by Olive Oil Phenolic Compounds in Humans. Free Radical Biology & Medicine. 2006;40(4):608-16.
- Vissers MN, Zock PL, Roodenburg AJ, Leenen R, Katan MB. Olive Oil Phenols Are Absorbed in Humans. The Journal of Nutrition. 2002;132(3):409-17.
- Miro-Casas E, Covas MI, Farre M, Fito M, Ortuno J, Weinbrenner T, Et Al. Hydroxytyrosol Disposition in Humans. Clinical Chemistry. 2003;49(6 Pt 1):945-52.
- Valls RM, Farras M, Suarez M, Fernandez-Castillejo S, Fito M, Konstantinidou V, Et Al. Effects of Functional Olive Oil Enriched with Its Own Phenolic Compounds on Endothelial Function in Hypertensive Patients. A Randomised Controlled Trial. Food Chemistry. 2015;167:30-5.
- Suarez M, Valls RM, Romero MP, Macia A, Fernandez S, Giralt M, Et Al. Bioavailability of Phenols from a Phenol-Enriched Olive Oil. The British Journal of Nutrition. 2011;106(11):1691-701.
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma Malondialdehyde as Biomarker for Oxidative Stress: Reference Interval and Effects of Life-Style Factors. Clinical Chemistry. 1997;43(7):1209-14.
- Tug T, Karatas F, Terzi SM, Ozdemir N. Comparison of Serum Malondialdehyde Levels Determined by Two Different Methods in Patients with COPD: HPLC or TBARS Methods. Laboratory Medicine. 2005;36(1):41-4.
- Bhutia Y, Ghosh A, Sherpa ML, Pal R, Mohanta PK. Serum Malondialdehyde Level: Surrogate Stress Marker in the Sikkimese Diabetics. Journal of Natural Science, Biology, and Medicine. 2011;2(1):107-12.
- Salami M, Galli C, De Angelis L, Visioli F. Formation of F2-Isoprostanes in Oxidized Low Density Lipoprotein: Inhibitory Effect of Hydroxytyrosol. Pharmacological Research. 1995;31(5):275-9.
- Yao Q, He G, Guo X, Hu Y, Shen Y, Gou X. Antioxidant Activity of Olive Wine, a Byproduct of Olive Mill Wastewater. Pharmaceutical Biology. 2016;54(10):2276-81.
- Bouwens M, Grootte Bromhaar M, Jansen J, Muller M, Afman LA. Postprandial Dietary Lipid-Specific Effects on Human Peripheral Blood Mononuclear Cell Gene Expression Profiles. The American Journal of Clinical Nutrition. 2010;91(1):208-17.