Blood transfusion practice: state of the art on promoting blood donation in Italy
Chiara Grecuccio1, Mariacarmela Ferraro1, Marco Colafelice1, Gaspare Adorno2, Giuseppe Liotta2, Leonardo Palombi2, Giuseppe Gervasi1
1School of Hygiene and Preventive Medicine, Department of Biomedicine and Prevention, University of Rome “Tor Vergata”
2Department of Biomedicine and Prevention, University of Rome “Tor Vergata”
Introduction
Blood transfusion is considered a longstanding public health issue worldwide. According to the World Health Organization (WHO), transfusion is one of the most important health interventions improving the quality of life of patients suffering of life-threatening diseases. Blood and blood products transfusions save millions of lives every year.
Safety is the most important concern related to blood transfusion. Besides safety, there are two other main challenges related to blood donations worldwide: obtaining adequate supply of blood, and reducing the striking difference in the level of blood accessibility between high- and low-income countries. Therefore, one of the goals of the WHO for 2020 is to obtain an adequate and safe blood supply for all countries in the world.1
WHO promotes activities devoted to increase the awareness about blood donation, through the support of appropriate public legislations.2 For this reason, the WHO wrote down the AIDE-Memoire for the Ministry of Health3 and the National Health Policy Makers,4 which are considered specific, easy and accessible guidelines or recommendations for donation activities.
The aim of the present study is to report about the state of the art of the activities related to the promotion of blood donation with particular attention to the Italian context.
General Background on Blood Donation
In 2008, the WHO collected reports from 164 countries (around 92% of world population) on blood donation and summarized it in the Global Database on Blood Safety (GDBS). The summary report 2011 on GDBS2 pointed out that around 92 million of blood units have been collected annually in 164 countries. Subsequently, WHO estimated the blood units gathered every year all over the world in about 103 million units.
The main indicator for general availability of blood in a country is the donation rate: no. of blood donors every thousand people. In high-income countries, the median donation rate is 33.1/1000 (range 13.3 – 64.6).5 However, recent data from WHO highlighted how in these countries only 10% of the eligible donors are currently donating. From 2008 to 2010, the total number of blood donations raised from 34.7 to 36.5 per 1000 inhabitants in the 30 countries of the WHO European Region (WHO-ER), with an average of blood donation range spanning from 6.0 to 67.6 per 1000 inhabitants.6
The easiest way to cover blood demand of each country clearly is to increase the number of donors worldwide. Moreover, in order to address its needs, each country must reach blood supplies self-sufficiency. Since blood units have a short shelf life, regular blood donors are essential to ensure a constant blood supply and to maintain national self-sufficiency. A regular donor is a subject that provides blood donations on a regular basis at least twice a year. According to this definition, WHO has estimated an average of 20-25 regular donors per 1000 inhabitants as the minimum threshold for each country to be self-sufficient. This quantity corresponds to an annual donation rate of 40 units per 1000 inhabitants.6
This goal presents many issues to be solved in several practical aspects. Among the others, the most important is the risk related to contagion of preventable transfusion transmissible infections (TTIs).7 Therefore, WHO has highlighted a prevalence of TTIs markers varying from 0.001% to 7.5% in donated blood unit samples. In particular, blood collected from subjects with particular habits (i.e. subjects having tattoos, sexual promiscuity, etc.) results repeatedly positive to HIV, HBV and HCV markers. Subsequently, these blood units shall be destroyed and not used for transfusion.
According to the donation programme, three different types of blood donors are commonly known: voluntary, familial/replacement or paid donors.5 Among these three groups, the voluntary donors present the lowest prevalence of TTIs markers. Today, however, less than 40% of blood supplies come from voluntary subjects. Voluntary and regular donors have demonstrated to be the safest blood suppliers.8
For these reasons, increasing the number of regular donors and therefore improving donors’ adherence are the most important issues to be addressed in a blood donation project.7
In Italy
In Italy blood donation is free, voluntary, anonymous and does not guarantee any personal benefit or profit,9,10 thus fitting the requirements of the WHO and the International Society of Blood Transfusion (ISBT).
Recently, the 2011 WHO report2 highlighted that only 10 countries over the world account for about 65% of the global blood supply. In this ranking, Italy is the 7th country in the world and the 3rd in Europe, with a donation rate of more than 40 blood units/1000 inhabitants.
Blood donation is important for the community and public health. Transfusion activities, as well as promotion of blood donation, are part of the Italian National Health Service (Servizio Sanitario Nazionale, SSN). Transfusions are guaranteed as part of the “Health Benefit Basket” (Livelli Essenziali di Assistenza, LEA). To this day, Italian donors between 18 and 65 years of age are around 1,700,000.11
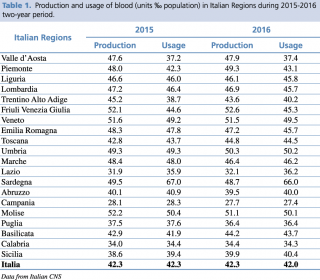
According to data provided by the National Institute of Health (Istituto Superiore di Sanità, ISS), Italy has been a self-sufficient country since 2010.12 Data from the National Blood Centre (NBC) highlight a peak of donations in Italy in 2012, with a production of 44.5 blood units per 1000 inhabitants. However, 2014 reported the lowest donation rate of about 42 blood units per 1000 inhabitants; the same rate reported for the following two years (2015 and 2016), as shown in Table 1.
Production and usage of blood (units ‰ population) in Italian Regions during 2015-2016 two-years period.
Furthermore, the 2015-2016 two-year period recorded an important non-uniformity in blood donation and transfusion in the 20 Italian regions.13 Interestingly, in 11 Italian Regions (i.e. Liguria, Lombardy, Trentino-Alto Adige, Veneto, Emilia Romagna, Marche, Sardinia, Abruzzo, Campania, Molise, Puglia) a negative trend of blood units production was evidenced. Blood units consumption showed a concomitant negative trend in 9 of these 11 regions, with a positive trend observed only in Veneto and Trentino Alto Adige. However, only Lombardy, Emilia Romagna, Campania, and Puglia, managed to guarantee sufficient supplies to cover their internal demand. In Sardinia and Abruzzo, a shortage of blood units remained during 2016, even though the data of NBC showed a reduction in blood usage. Finally, Lazio and Sicilia regions showed a contemporary reduction of both usage and collection of blood. However, the local need is not satisfied because blood usage is anyway greater than local production.13 This data clarifies why 4/20 Italian regions need to request blood units from other regions, resulting in higher internal expenses and important delays in clinical practices.
These data explain well the difficulties faced in recruiting new donors during the last 5 years, in particular young people necessary to guarantee the generational replacement. This replacement is important since the negative trend of the Italian demography contributes to lowering the number of young donors, namely subjects between 18-35 years of age. Today, most of the donors come from population between 35-55 years of age, that will decrease in the next decades according to the demographic projections. In fact, in 2015, young donors were respectively 13.39% (18-25 age range) and 18.28% (26-35 age range) of all Italian donors, with an overall of 31.67% for subjects between 18 and 35 years old. These percentages could not be acceptable if we consider data about the ageing of actual donors and their subsequent estimated decrease of 4.5% for the 2020.11 In consideration of these demographic modifications, collaboration between the National Blood Centre (NBC) and the non-profit Associations is necessary in order to guarantee an adequate generational turnover of donors.
Donor Adherence
Increasing the number of donors and maintaining their constant participation to donation programs is the final aim of all non-profit associations dealing with transfusions worldwide.
A National blood network is considered the best solution to guarantee high standards of quality and safety in blood and blood product collection. Since 1975, WHO promoted the development of national blood networks based on voluntary non-remunerated donors. In 2010, WHO addressed the WHA 63.12 resolution urging all Member States to develop a national blood network based on voluntary and unpaid donors.5 WHO recommended that every country should put in force the necessary policies, systems and structures to build its own national blood network.
Moreover, each country is responsible to ensure safe, adequate and available blood supplies covering its national needs. Thus, writing a National Blood Policy and Plan (NBPP) could help to optimize blood transfusion in terms of efficacy, safety and costs. Today, only 35% of WHO Member States implemented a national blood policy, relevant legislation and specific organizations for blood donation services.8 Each NBPP must define the national principles in blood donation. Furthermore, NBPP must coordinate functions, organization and management of each Blood Transfusion Service (BTS). A BTS is a service set up to manage and to collect blood donations. BTS promotes the adherence to high quality standards, minimizing duplications and achieving economies of scale and cost advantages through the implementation of a national system for recruitment, screening and processing of donors. A well-organized BTS is worldwide considered better than a hospital-based system in terms of cost-effectiveness and safety.8
For this reason, national health authorities must support BTS activities through adequate legislation and regulation.
Italian Regulatory Framework
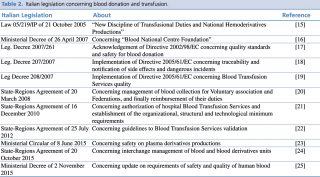
In Italy, a National Transfusion Network was set up in 2005. Each BTS belongs to NHS and they cooperate with each other to reach regional and national self-sufficiency.14 The Italian National Transfusion Network laws and regulations are presented in Table 2.15-25
Italian legislation concerning blood donation and transfusion.
Since 2005, Italy recognizes blood donation as an ethic and civic function regulated by the law (i.e. L. 05/219/IP). The main topics covered by this law are:
-
Guidelines of quality and safety for blood donation and BTS healthcare;
-
The “Health Benefit Basket” (Livelli Essenziali di Assistenza, LEA) in transfusion medicine;
-
Identification of national and regional coordination system;
-
Revision of accreditation requirements.
The quality standards and safety requirements for blood donation were set up by the regional coordination system (Centro Regionale Sangue, CRS). The main purpose of CRS is to reach the national self-sufficiency. Moreover, the law L. 05/219/IP defines the role and features of associations of voluntary donors that contribute to promote and also to develop a donation culture.15
The National Blood Centre (Centro Nazionale Sangue, CNS) was established on the 1st of August 2007 inside the ISS (Istituto Superiore di Sanità). CNS addresses several duties, such as supporting BTS planning, scientific research, and control and supervision of the national transfusion network.16
In 2007, the Italian Parliament implemented the European Recommendation on blood donation (2002/98/EC) that establishes precise standards of quality and safety. BTS should play a crucial role in collecting, testing, processing, storing and distributing human blood and human components.17 BTS should allow the traceability and notification of adverse events. BTS also must guarantee traceability of human blood and blood products, through identification procedures, and the proper use of registries and rating plates.18 A key-step is the first contact with the potential donor, in which the physician gives a judgement of eligibility. Human blood units and blood components must respect precise procedures and requirements, as the cleanliness of blood sacks. All aspects of quality and safety of blood donation are presented in the Legislative Decree 208/2007.19
Since 2010, in Italy, a hospital eligible to set up a BTS can be authorized in Italy only if specific organizational, structural and technological requirements are met. Each two-year period NBC must guarantee the validation process of each hospital BTS. Moreover, medical and nursing staff working in blood collection in a BTS must possess a specific certificate, which states the acquirement of specific operational capabilities and skills.21 Besides hospital BTS, also local BTS and temporarily collection unit of blood and blood components must be authorized through specific national guidelines.22
In 2015, the Minister of Health, through a ministerial circular (MC), presented the criteria to improve safety on national plasma and medical plasma derivatives production.23 This MC introduced the Nucleic Acid Test (NAT) for both Hepatitis A virus (HAV) and Parvovirus B19 carried out on mini pools of plasma. Aim of this MC was to ensure that the national plasma derivatives production reached the same level of safety and efficacy of the European productions. In the same year, interchange managements of blood units and blood components (such as immunoglobulin, albumin, coagulation factors and other plasma derivate products) were regulated between hospitals and the Italian Regions. In particular, standard incentive measures and prices for blood units and blood components were established.24
Finally, a Ministerial Decree (MD) updated the requirements on safety and quality of human blood. This MD established all the procedures that have to be followed in the different phases of blood donation and blood transfusion, from information to eligible donors to blood transfusion procedures and its possible adverse events.25
Discussion and Conclusion
Blood transfusion is an important medical procedure, useful for managing several clinical life-threating conditions.
However, data from GBDS pointed out that sometimes transfusion is provided also when therapeutic alternatives are possible.2 For this reason, Liumbruno et al.26 raised the following question: “To transfuse or not to transfuse?”. A recent meta-analysis27 showed a reduced OR for mortality in patients transfused with a threshold of 10 gr/dL. However, a sub-analysis presented in the same paper did not confirm the above-mentioned results when only high-quality studies were taken into account.
The recent debate on transfusion thresholds, in fact, is one of the public health issues to be addressed, mainly concerning the safety and usefulness of a conservative transfusion approach versus a liberal one, consisting respectively in a threshold of 8 and 10 gr/dL haemoglobin. The discussion is about the safety of patients transfused, with particular attention to adverse events related to the transfusion, such as TTI, blood group associated haemolysis, and circulatory overload. Authors concluded that blood transfusion should be given only in patients with 7-8 gr/dL haemoglobin values with concomitant symptoms of anaemia. A threshold of 10 gr/dL could be considered only in case of patients presenting symptoms and signs of ischemic heart disease.
Nevertheless, the GBDS report that 40 countries include more than 47,000 hospitals performing blood transfusion, serving a population of around 4.2 billion people. In 2006, data from 90 countries accounted for more than 9 million blood transfusion performed, with a relevant difference between high- and low-income countries. In developing countries, transfusion is used more often in preventing pregnancy-related complications, and for severe childhood anaemia treatment. In developed countries, instead, blood transfusion is most commonly used for prevention and care of patients with severe chronic diseases (i.e. cardiovascular disorders, solid and haematological tumours), especially in the elderly age.2 Interestingly, data from an Italian hospital in Rome showed how anaemia could prolong the hospital length of stay (LOS). In particular, subjects over 65 years old presenting anaemia showed a mean LOS of 14.3±11.0 days (mean±1DS), while the average LOS for all ages was 9.84±9.65 days (data not published).
Recently, pharmaceutical industries approach to blood transfusion promoted the use of the so-called “artificial blood”. These “synthetic” products are oxygen carriers, with a half-life of around 30 hours. The clinical use of these molecules is still yet to be tested to assess their efficacy.28 However, the relative lower half-life highlighted the importance of human blood transfusion in therapeutic approaches. Moreover, blood products have been used also in the prevention and care of clinical conditions such as bedsore or haemorrhagic cystitis.
That said, the importance of blood donation and the preventive action of blood transfusion in several clinical practices is clear. Two conditions are essential in promoting blood donation: spreading the feeling of being part of a community and the realization of a culture based on sharing with others. For this purpose, every regional agency plays an important role through educational and formative activities. Therefore, the non-profit associations of voluntary donors play an important role in the transfusion network, as they take care of the promotion and the development of organized blood donations as well as to ensure donors protection. These concepts are well defined through the planning of several campaigns pro-donation. For this reason, each 14th June, the WHO organizes and manages the so-called “World Blood Donor Day”. During that day, every country present its contribution in promoting blood donation, and finally, new regional promotional campaigns are planned.1 Moreover, each BTS must be aware and proud of giving a tangible contribution to the improvement of blood safety. For this reason, the WHO has proposed several programmes of distance learning in blood safety. Each BTS can download learning material from WHO website to manage and build learning programmes for clinicians and health operators.29
Nowadays, the reduced social expenditure, associated with the reduction of social relationships and belonging feelings, is deteriorating the society. In this context, it is important to create synergic action to favour and promote a better social “tissue”. This could represent a relevant benefit for blood donation campaigns.
In this perspective, young people have to realise that everyone is part of the community and each person can make the difference playing its part in blood donation. This choice will have positive consequences at every level in an evolving society.
References
-
Global Database on Blood Safety. Summary report 2011. [Internet] Geneva. World Health Organization. 2011 [cited May 2017]. Available from http://www.who.int/bloodsafety/global_database/GDBS_Summary_Report_2011.pdf?ua=1
-
ISBT Code of Ethics; Third draft (1999). Transfusion today, 36, 21-22 June 1999
-
Decreto legislativo del 28 Giugno 2016. Misure per lo sviluppo della produzione e dell'utilizzazione dei prodotti derivati dal sangue o dal plasma umani provenienti da donazioni volontarie e non remunerate. Gazzetta Ufficiale 2016-GU-185-09/08/2016. Available from http://www.centronazionalesangue.it/pagine/autosufficienza
-
Ministero della salute, Direzione generale della digitalizzazione, del sistema informativo sanitario e della statistica. Rete trasfusionale. [Internet]. Roma. Ministero della salute, Direzione generale della comunicazione e dei rapporti europei e internazionali. 2014. [cited May 2017] Available from http://www.salute.gov.it/portale/temi
-
Italian Minister of Health. [Sistema Trasfusionale]. Available from http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=2932&area=sangueTrasfusioni&menu=trasfusionale
-
Decreto Ministeriale DM 2 novembre 2015, avente per oggetto “Disposizioni relative ai requisiti di qualità e sicurezza del sangue e degli emocomponenti.”
-
Sarkar S. Artificial Blood. Indian J Crit Care Med. 2008;12(3):140-4.
- World Health Organization, Blood Transfusion Safety. Distance Learning in Blood Safety. A Flexible, Cost-Effective Approach to Staff Development in Blood Transfusion Services. [Internet] Geneva. WHO; 2017 [cited May 2017]. Available from http://apps.who.int/medicinedocs/documents/s15396e/s15396e.pdf