Work-Related Allergic Rhinitis: a contemporary re-view of the literature
Maria Paola Balla,1 Cataldo Marsico,2 Serafino Ricci,1 Lidia Ricci,1 Salvatore Marsico,3 Pasquale Ricci,1 Francesco Massoni,1 Massimo Ralli4
1 Department of Anatomy, Histology, Legal Medicine and Orthopedics, Sapienza University of Rome, Italy
2 Sant’Eugenio Hospital, Rome, Italy
3 Istituto Nazionale Previdenza Sociale, Rome, Italy
4 Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, Italy
Introduction
Allergological risk has gained in the recent years an ever-increasing importance in professional respiratory disease; substances capable of causing allergic respiratory diseases are more numerous than those that can cause pneumoconiosis, thus leading to bronchial asthma, the most fearsome professional respiratory disease.1-3 As a result, the interest of occupational medicine has also increased for work-related allergic rhinitis (WRAR), an entity which was considered to be of little clinical interest in the past.4-6
WRAR is a chronic inflammatory disease of the upper respiratory tract with allergic basis characterized by persistent or recurrent symptoms such as itching, sneezing, and nasal congestion and anterior rhinorrhea, and can be associates to other respiratory illnesses, such as inflammation of paranasal sinuses, olfactory disorders, bronchial asthma,7,8 and other generic clinical conditions, such as headaches or conjunctivitis. In WRAR, symptoms occur only after exposure to specific factors in the working environment and significantly affect the quality of life and work performance of workers.5-7 In many cases, WRAR precedes or accompanies the onset of bronchial hyper responsiveness, and acts as the first indicator of an allergy in the respiratory system. This allows workers to notice the first symptoms of allergic rhinitis from a particular working environment before the onset of professional bronchial asthma.9-11
This work is aimed at providing an update on epidemiology, pathogenesis, clinical symptoms, diagnosis and treatment of WRAR, also presenting a personal case series on 1402 patients.
Epidemiology
The burden of rhinitis in the adult population is estimated between 10 and 30%.12-14 The International Study on Asthma and Allergies in Childhood (ISAAC) showed a prevalence in children between 0.8% and 14.9% in 6-7 year olds and between 1.4% and 39.7% in 13-14 year olds.15 There is no equivalent to ISAAC for adults; national surveys show prevalence rates of rhinitis of between 5.9% and 29% with a mean of 16%. Perennial rhinitis is probably more common in adults than in children.12-14
The overall incidence of professional rhinitis has been estimated on about 7-12% of the general working population;4-6 according to Siracusa, WRAR has an incidence of 2-87% of workers exposed to high-molecular weight agents (HMW) and 3-48% of workers exposed to low-molecular weight agents (LMW).16 However, overall prevalence of rhinitis related to workspace if probably underestimated due to the heterogeneous diagnostic criteria used by different researchers.17
Pathophysiological Mechanisms
The pathophysiological mechanisms underlying allergic rhinitis are started by an inflammatory response in the nasal mucosa composed of a rapid immunoglobulin-E(IgE)-mediated mast cell response followed by a late-phase response in which eosinophils, basophils and T cells play a central role. Involved T cells have been shown to express a Th2 cytokine profile with interleukin (IL)-4 and IL-5; cytokines regulating the Th2 response have been recently shown in the allergic rhinitis pathogenic mechanisms.18
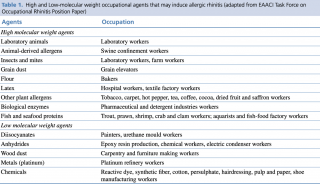
Allergens at the basis of WRAR can be divided into HMW and LMW agents. The former are more relevant in WRAR as their pathophysiologic mechanisms are based on the capacity of agents to induce immune responses through IgE dependent reactions. LMW agents’ mechanisms of action are less known and have been shown to induce allergic response through other mechanisms such as non-immunomediated degranulation of mast cells.3,8,18 High and Low-molecular weight occupational agents that may induce WRAR are listed in Table 1.
Table 1. High and Low-molecular weight occupational agents that may induce allergic rhinitis (adapted from EAACI Task Force on Occupational Rhinitis Position Paper)8
There are many humoral and cellular immune mechanisms in the background of WRAR, which are caused by exposure to allergens in the work environment and lead to specific hypersensitivity. Symptoms usually appear after latency in predisposed individuals and return after each subsequent exposure or persist continuously, depending on the frequency of exposure.19,20
The latency between initial exposure and first symptoms of WRAR has been reported to range from 1 month to 18 years, with an average of 2-3 years.21 When sensitized, nasal symptoms occur within minutes after exposure for HMW allergens and to up to several hours for LMW allergens.7 The development of WRAR is dependent on the level and duration of exposure to allergens.22
Clinical Symptoms
WRAR symptoms are the same of non-work related allergic rhinitis, and include nasal mucus, rhinorrhea, cough, pruritus and reduced nasal flows following exposure to allergens. A reduction of the sense of smell has also been often reported in WRAR with high impact on quality of life (QoL), although very little has been published about the combination of olfactory impairment, allergic rhinitis and QoL.23 Symptoms can be worsened in a workplace environment by a variety of irritating factors, such as chemical vapors, dust, fluids and non-physiologic environmental temperature exposure.4,5,21
The development of WRAR is also linked to the duration and magnitude of exposure to allergens, mostly HMW; such allergens, although may be present also outside the working environment, have a much higher concentration in working environments.4,6,7,16 Symptoms are also related to individual factors such as sex and age.24 Atopy is the main factor playing a role in individual responsiveness to allergen exposure, and acts as a predisposing factor toward developing certain allergic hypersensitivity reactions; most reports refer to the role of atopy as a risk factor for the development of occupational asthma mainly in terms of exposure to HMW. Its association with the development of occupational rhinitis has been demonstrated in several studies.24-29
Unrecognized occupational exposure may lead to resistance to treatment and to development of complications such as nasal polyposis, middle ear chronic diseases, lower respiratory tract infections, and sleep disorders.16,30-33
The Association with Asthma
A strong association has been demonstrated between WRAR and occupational asthma (OA).9-11,34,35 Many authors have shown that rhinitis is an independent risk factor for bronchial asthma in both atopic and non-atopic individuals, and that asthma risk increases with duration and symptom severity.10,27,28,36 Data from the literature indicate that the number of WRAR cases is 2-4 times greater than OA;21 Malo reported that most patients diagnosed with OA also suffer from occupational rhinitis; the prevalence of symptoms was not different for HMW and LMW agents, although rhinitis was more intense for HMW than for LMW.37
Other studies confirm that the presence of WRAR increases the risk of developing OA; the importance of this association is in early diagnosis and prevention of secondary OA.9,34,35 A prospective study on natural history after professional rhinitis by Karjalainen showed that during the 12-year follow-up, 11.6% of the patients with established work-related rhinitis developed OA, compared to 3.1% of the control group.38
Diagnosis
The diagnosis of WRAR is based on the demonstration of typical rhinitis symptoms that are exacerbated in the workplace environment, thus proving its association with occupational exposure, and excluding other conditions that may produce similar symptoms. Diagnosis is based on anamnestic interview, workplace environment study, clinical and instrumental examination, nasal cytology, and specific allergological tests.1,3,5
Anamnesis and workplace examination include analysis of medical documentation and interview with patient and, possibly, employer, with the aim to investigate individual medical history and timing and degree of exposure.5,39
Nasal examination includes rhinoscopy, with assessment of nasal septum and mucous membranes, evaluating macroscopic changes in the nasal mucosa and, especially, in the lower turbinate area. Instrumental evaluation of nasal flows should be performed through rhinomanometry, to assess nasal resistances and air flow volumes.40-42
Nasal cytology has a role in determination of allergic nature of a rhinitis, assessing the presence of inflammatory cells and markers within the nasal mucosa and its secretions;43-46 however, alterations in cytology may be present in non-allergic conditions causing nasal inflammation.44
Testing for reaction to specific allergens can be helpful in confirming the diagnosis of allergic rhinitis and in the determination of specific allergic triggers.5,17,42 Allergy skin tests are an in vivo method to determine immediate IgE-mediated hypersensitivity to specific allergens.47-50
In vitro allergy tests allow measurement of the amount of specific IgE to individual allergens in a blood sample, correlating the amount of specific IgE produced to a particular allergen to the allergic sensitivity to that factor. The measurement of the total level of IgE in the blood is neither sensitive nor specific for allergic rhinitis.22,48,51,52 Immunological tests are part of the diagnostic procedure for WRAR, although may return false positive results and, as previously stated, are not unique indicators of the workplace exposure to allergens.40,53-55 Serum IgE assay is a relevant test in allergy examination; however, the results from previous studies showed no significant differences in allergen-specific IgE concentrations in workers compared to controls who were not previously exposed to organic dust. Therefore, allergen molecular diagnostics may represent a useful test in allergy diagnostic process, but deserves caution in particular circumstances.2,40,53-58
Inhaled or intranasal tests using professional allergens are the gold standard in WRAR diagnosis. They can be performed at work or in laboratory and represent an objective method for assessing the presence of WRAR and the type of allergen at its base.1,5,13,49
Treatment
The most effective therapy for WRAR is avoidance or reduction of exposure by workers to allergens that have caused it. This can be accomplished through workplace interventions, such as replacement of causative substances, the use of environment ventilation and personal protective equipment, and changing worker’s duties. Also, a role is played by education of employees and employers.2,18,40,59,60
Pharmacological treatment is no different from that of non-professional allergic rhinitis17,60 and should be complementary to workplace intervention. Pharmacotherapy is based on the administration of antihistamines and on the use of nasal corticosteroids, and only in the most serious cases, of systemic corticosteroid cycles.
The specific immunotherapy, commonly used in allergic rhinitis, is only indicated in certain types of WRAR such as allergy to laboratory animals.61-64
Our Clinical Experience
We conducted a retrospective analysis on 1402 consecutive patients presenting to the Otolaryngology service of the S. Eugenio Hospital in Rome, Italy from July 2005 to July 2016 with a diagnosis of allergic rhinitis. The diagnostic protocol included an accurate history, physical examination and rhinoscopy with the use of nasal fibroscopy, and skin allergy tests and of the stop-recovery test in case of negativity of previous tests.
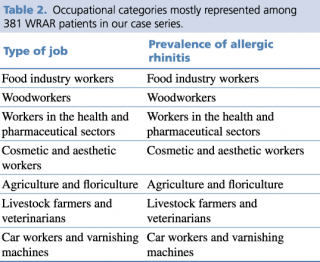
Out of 1402 cases with a diagnosis of allergic rhinitis, 381 (27.2%) were found to have a correlation with workplace environment allergens, thus were related to occupational activity and diagnosed with WRAR. Occupational categories mostly represented among WRAR patients are listed in Table 2. In details, the sample included 142 (37.4%) employees in the food industry (food, catering, mill workers, bakers, pastry makers); 57 (15.1%) woodworkers (industry and crafts); 46 (12.2%) employees in the health and pharmaceutical sector; 42 (11.1%) cosmetics (industry, hairdressers, aesthetics); 39 (10.1%) agriculture and floriculture (production and sales); 40 (10.2%) veterinarians, and 15 (3.9%) bodyworkers and varnishing machines.
Table 2. Occupational categories mostly represented among 381 WRAR patients in our case series.
Conclusions
Prevention is the key element in WRAR, including a range of pro-therapeutic activities at both the primary and secondary prevention stages. These should include interventions aimed to reduce the level of exposure of workers to etiologic factors, such as environmental changes in the workplace, the use of appropriate protective equipment, and the information among employees about presence, symptoms and risks of WRAR. Medical surveillance to determine the individual risk factors for development of WRAR should be considered. The most effective therapy of WRAR is avoidance or reduction of exposure by workers to allergens that have caused it.
Another aspect worth investigation is assessment of QoL in WRAR. It has been proved that the QoL deteriorates after, for example, the onset of a loss of smell that often follows WRAR, and this include adverse effects on appreciation of food, interference with daily routine, physical health, profession, emotional stability, leisure and a general worsening in well-being. Very little has been published about the combination of olfactory impairment, allergic rhinitis and QoL; this encourages further studies in the field especially focusing on WRAR.
It is therefore important to adequately assess, communicate and manage risks in occupational chemical exposure settings with the aim to protect workers and the necessity to introduce periodic health examinations programs focusing on workers to monitor health and well-being and improve working conditions and the working environment.
Disclosures
The following paper has been presented as a poster (Poster 2.13) to the 1st Scientific International Conference on CBRNe.
References
-
Hamizan AW, Rimmer J, Alvarado R, Sewell WA, Kalish L, Sacks R, et al. Positive Allergen Reaction in Allergic and Nonallergic Rhinitis: A Systematic Review. Int Forum Allergy Rhinol. 2017.
-
Reichmuth D, Lockey RF. Present and Potential Therapy for Allergic Rhinitis: A Review. BioDrugs. 2000;14(6):371-87.
-
Walker CL. Allergic Rhinitis: A Review. ComprTher. 1992;18(3):11-3.
-
Gervais P, Ghaem A, Eloit C. Occupational Allergic Rhinitis. Rhinology. 1985;23(2):92-8.
-
Gomez F, Rondon C, Salas M, Campo P. Local Allergic Rhinitis: Mechanisms, Diagnosis and Relevance for Occupational Rhinitis. CurrOpin Allergy ClinImmunol. 2015;15(2):111-6.
-
Shusterman D. Occupational Irritant and Allergic Rhinitis. Curr Allergy Asthma Rep. 2014;14(4):425.
-
Hox V, Steelant B, Fokkens W, Nemery B, Hellings PW. Occupational Upper Airway Disease: How Work Affects the Nose. Allergy. 2014;69(3):282-91.
-
EAACI Task Force on Occupational Rhinitis, Moscato G, Vandenplas O, Gerth Van Wijk R, Malo JL, Quirce S, Walusiak J, Castano R, De Groot H, Folletti I, Gautrin D, Yacoub MR, Perfetti L, Siracusa A. Occupational Rhinitis. Allergy. 2008 Aug;63(8):969-80.
-
Bonavia M, Crimi E, Quaglia A, Brusasco V. Comparison of Early and Late Asthmatic Responses Between Patients with Allergic Rhinitis and Mild Asthma. EurRespir J. 1996;9(5):905-9.
-
Kellberger J, Peters-Weist AS, Heinrich S, Pfeiffer S, Vogelberg C, Roller D, et al. Predictors of Work-Related Sensitisation, Allergic Rhinitis and Asthma in Early Work Life. EurRespir J. 2014;44(3):657-65.
-
Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, et al. Specific Patterns of Allergic Sensitization in Early Childhood and Asthma & Rhinitis Risk. ClinExp Allergy. 2013;43(2):233-41.
-
Mims JW. Epidemiology of Allergic Rhinitis. Int Forum Allergy Rhinol. 2014;4 Suppl2:S18-20.
-
Settipane RA, Charnock DR. Epidemiology of Rhinitis: Allergic and Nonallergic. Clin Allergy Immunol. 2007; 19:23-34.
-
Sly RM. Epidemiology of Allergic Rhinitis. Clin Rev Allergy Immunol. 2002;22(1):67-103.
-
Shah AA. International Study of Asthma and Allergies in Childhood (ISAAC). J Assoc Physicians India. 1994;42(3):265.
-
Siracusa A, Desrosiers M, Marabini A. Epidemiology of Occupational Rhinitis: Prevalence, Aetiology and Determinants. ClinExp Allergy. 2000;30(11):1519-34.
-
Hellgren J, Karlsson G, Toren K. The Dilemma of Occupational Rhinitis: Management Options. Am J Respir Med. 2003;2(4):333-41.
-
Incorvaia C, Fuiano N, Martignago I, Gritti BL, Ridolo E. Local Allergic Rhinitis: Evolution of Concepts. ClinTransl Allergy. 2017 Nov 2; 7:38.
-
Broide DH. Allergic Rhinitis: Pathophysiology. Allergy Asthma Proc. 2010;31(5):370-4.
-
Sin B, Togias A. Pathophysiology of Allergic and Nonallergic Rhinitis. Proc Am Thorac Soc. 2011;8(1):106-14.
-
Ruoppi P, Koistinen T, Susitaival P, Honkanen J, Soininen H. Frequency of Allergic Rhinitis to Laboratory Animals in University Employees as Confirmed by Chamber Challenges. Allergy. 2004;59(3):295-301.
-
Storaas T, Steinsvag SK, Florvaag E, Irgens A, Aasen TB. Occupational Rhinitis: Diagnostic Criteria, Relation to Lower Airway Symptoms and Ige Sensitization in Bakery Workers. ActaOtolaryngol. 2005;125(11):1211-7.
-
Passali GC, Ralli M, Galli J, Calo L, Paludetti G. How Relevant Is the Impairment of Smell for the Quality of Life in Allergic Rhinitis? CurrOpin Allergy ClinImmunol. 2008;8(3):238-42.
-
Shusterman D, Murphy MA, Balmes J. Differences in Nasal Irritant Sensitivity by Age, Gender, and Allergic Rhinitis Status. Int Arch Occup Environ Health. 2003;76(8):577-83.
-
Chan EY, Ng DK, Chan CH. Measuring FENO in Asthma: Coexisting Allergic Rhinitis and Severity of Atopy as Confounding Factors. Am J RespirCrit Care Med. 2009;180(3):281; author reply 2.
-
Fuiano N, Incorvaia C. Utility of the Atopy Patch Test in the Diagnosis of Allergic Rhinitis. Iran J Otorhinolaryngol. 2016;28(86):169-75.
-
Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an Independent Risk Factor for Adult-Onset Asthma. J Allergy ClinImmunol. 2002;109(3):419-25.
-
Jang AS, Kim SH, Kim TB, Park HW, Kim SH, Chang YS, et al. Impact of Atopy on Asthma and Allergic Rhinitis in the Cohort for Reality and Evolution of Adult Asthma in Korea. Allergy Asthma Immunol Res. 2013;5(3):143-9.
-
Wang de Y. Genetic Predisposition for Atopy and Allergic Rhinitis in the Singapore Chinese Population. Asia Pac Allergy. 2011;1(3):152-6.
-
SacreHazouri JA. [Allergic Rhinitis. Coexistent Diseases and Complications. A Review and Analysis.] Rev Alerg Mex. 2006;53(1):9-29.
-
Settipane RA. Complications of Allergic Rhinitis. Allergy Asthma Proc. 1999;20(4):209-13.
-
Skoner DP. Complications of Allergic Rhinitis. J Allergy ClinImmunol. 2000;105(6 Pt 2): S605-9.
-
Slavin RG. Complications of Allergic Rhinitis: Implications for Sinusitis and Asthma. J Allergy ClinImmunol. 1998;101(2 Pt 2): S357-60.
-
Gautrin D, Malo JL. Risk Factors, Predictors, and Markers for Work-Related Asthma and Rhinitis. Curr Allergy Asthma Rep. 2010;10(5):365-72.
-
Vandenplas O, Van Brussel P, D'Alpaos V, Wattiez M, Jamart J, Thimpont J. Rhinitis in Subjects with Work-Exacerbated Asthma. Respir Med. 2010;104(4):497-503.
-
Khan DA. Allergic Rhinitis and Asthma: Epidemiology and Common Pathophysiology. Allergy Asthma Proc. 2014;35(5):357-61.
-
Malo JL, Lemiere C, Desjardins A, Cartier A. Prevalence and Intensity of Rhinoconjunctivitis in Subjects with Occupational Asthma. EurRespir J. 1997;10(7):1513-5.
-
Karjalainen A, Martikainen R, Klaukka T, Saarinen K, Uitti J. Risk of Asthma among Finnish Patients with Occupational Rhinitis. Chest. 2003;123(1):283-8.
-
Wiszniewska M, Walusiak-Skorupa J. Diagnosis and Frequency of Work-Exacerbated Asthma Among Bakers. Ann Allergy Asthma Immunol. 2013;111(5):370-5.
-
Fujieda S. [Diagnostic and Treatment of Allergic Rhinitis]. Nihon Jibiinkoka Gakkai Kaiho. 2013;116(2):110-3.
-
Mancilla-Hernandez E, Medina-Avalos MA, Osorio-Escamilla RE. [Validation of a Diagnostic Questionnaire of Allergic Rhinitis in Children and Adults for Epidemiological Studies]. Rev Alerg Mex. 2014;61(3):153-61.
-
Sperl A, Klimek L. [Diagnostic Methods of Allergic Rhinitis]. Med Monatsschr Pharm. 2016;39(3):100-4.
-
Chen J, Zhou Y, Zhang L, Wang Y, Pepper AN, Cho SH, et al. Individualized Treatment of Allergic Rhinitis According to Nasal Cytology. Allergy Asthma Immunol Res. 2017;9(5):403-9.
-
Gelardi M, Incorvaia C, Fiorella ML, Petrone P, Quaranta N, Russo C, et al. The Clinical Stage of Allergic Rhinitis is Correlated to Inflammation as Detected by Nasal Cytology. Inflamm Allergy Drug Targets. 2011;10(6):472-6.
-
Meltzer EO, Orgel HA, Rogenes PR, Field EA. Nasal Cytology in Patients with Allergic Rhinitis: Effects of Intranasal Fluticasone Propionate. J Allergy ClinImmunol. 1994;94(4):708-15.
-
Ozgur A, Arslanoglu S, Etit D, Demiray U, Onal HK. Comparison of Nasal Cytology and Symptom Scores in Patients with Seasonal Allergic Rhinitis, before and after Treatment. J Laryngol Otol. 2011;125(10):1028-32.
-
Jeng KC, Hsaio SH, Liu MT, Wang JS. The Relationship of Ige, Skin-Test, Eosinophilia, Eosinophil Cationic Protein and Tumor Necrosis Factor Production in Allergic Rhinitis. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi XueZaZhi. 1991;24(4):345-54.
-
Fromer L. Diagnosing Allergic Rhinitis: Skin Test vs. Ige Testing. Am Fam Physician. 2006;73(9):1517.
-
Kim BJ, Mun SK. Objective Measurements Using the Skin Prick Test in Allergic Rhinitis. Arch Otolaryngol Head Neck Surg. 2010;136(11):1104-6.
-
Ta V, Scott DR, Chin WK, Wineinger NE, Kelso JM, White AA. Differential Skin Test Reactivity to Pollens in Pollen Food Allergy Syndrome versus Allergic Rhinitis. Allergy Asthma Proc. 2015;36(5):379-85.
-
Pumhirun P, Jane-Trakoonroj S, Wasuwat P. Comparison of In Vitro Assay for Specific Ige and Skin Prick Test with Intradermal Test in Patients with Allergic Rhinitis. Asian Pac J Allergy Immunol. 2000;18(3):157-60.
-
Wei X, Fu Z, Lin X, Li J, Deng H, Li H, et al. [Comparison of Serum Specific Ige and Skin Prick Test in Allergic Rhinitis Patients Sensitive to Dust Mite]. Lin Chung Er Bi Yan HouTou Jing Wai KeZaZhi. 2013;27(8):404-6.
-
Berman BA, Ross RN. Conversations on Allergy and Immunology. Allergic Rhinitis. Cutis. 1983;31(5):458-64, 70.
-
Hatzitheodorou G, Palaiologos Y, Simascos N, Haviaras V. [Immunology of Allergic Rhinitis]. Rev LaryngolOtolRhinol (Bord). 1980;101(11-12):499-502.
-
Lund VJ, Wright DJ, Davies RJ. Immunology of Allergic Rhinitis, or a Nose for Treatment. J R Soc Med. 1986;79(10):618-21.
-
Bousquet J, Van Cauwenberge P, Bachert C, Canonica GW, Demoly P, Durham SR, et al. Requirements for Medications Commonly Used in the Treatment of Allergic Rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and Its Impact on Asthma (ARIA). Allergy. 2003;58(3):192-7.
-
Cingi C, Gevaert P, Mosges R, Rondon C, Hox V, Rudenko M, et al. Multi-Morbidities of Allergic Rhinitis in Adults: European Academy of Allergy and Clinical Immunology Task Force Report. ClinTransl Allergy. 2017; 7:17.
-
Okubo K. [Clinical Investigation of Allergic Rhinitis: Pathophysiology and Immunology]. Arerugi. 2014;63(10):1317-24.
-
Szeinbach SL, Seoane-Vazquez EC, Beyer A, Williams PB. The Impact of Allergic Rhinitis on Work Productivity. Prim Care Respir J. 2007;16(2):98-105.
-
van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus Statement on the Treatment of Allergic Rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(2):116-34.
-
Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen Immunotherapy for Allergic Rhinoconjunctivitis: A Systematic Review and Meta-Analysis. Allergy. 2017.
-
Mortuaire G, Michel J, Papon JF, Malard O, Ebbo D, Crampette L, et al. Specific Immunotherapy in Allergic Rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):253-8.
-
Nurmatov U, Dhami S, Arasi S, Roberts G, Pfaar O, Muraro A, et al. Allergen Immunotherapy for Allergic Rhinoconjunctivitis: A Systematic Overview of Systematic Reviews. ClinTransl Allergy. 2017; 7:24.
-
Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, et al. Biomarkers for Monitoring Clinical Efficacy of Allergen Immunotherapy for Allergic Rhinoconjunctivitis and Allergic Asthma: An EAACI Position Paper. Allergy. 2017;72(8):1156-73.