Dual-use Molecules from Yeast
Angela Cirigliano1 (orcid.org/0000-0003-0790-4638), Rodolfo Negri1 (orcid.org/0000-0002-4806-7090) and Teresa Rinaldi1,2* (orcid.org/0000-0001-6291-245X)
1 Department of Biology and Biotechnology “Charles Darwin”, La Sapienza University of Rome, Italy
2 Italian Army, Ministry of Defence, Italy
Introduction
This year the OPCW, the implementing body for the Chemical Weapons Convention, celebrates the 20th anniversary of entry into forces. In 2014, this organization examined the impact of new technologies in the field of chemical and biological weapons, in particular the “Convergence” of Chemistry and Biology. An OPCW report of the Scientific Advisory Group highlighted the importance of monitoring developments in science and technology: “New production processes, combined with developments in drug discovery and delivery, could be exploited in the development of new toxic chemicals that could be used as weapons.”1 Indeed, since 2008, Synthetic Biology is monitored also by other international organizations, such as the Nonproliferation Export Control Regimes Australia Group.2,3
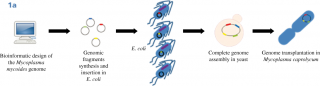
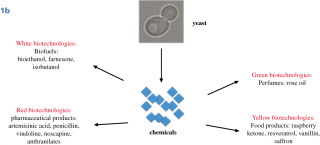
Until now, the interest in Synthetic Biology research as a proliferation risk,4,5,6 was essentially based on the possibility to create from scratch lethal viruses; indeed, whole bacterial genomes can be now synthesized in laboratories and assembled in yeast cells and non-essential genes can be reshuffled and/or eliminated from the genome. A synthetic yeast genome itself is under construction,7,8,9,10 (this field of Synthetic Biology is characterized by a bottom-up approach, Fig.1A). This achievement is considered a “Convergence” of Chemistry towards Biology because it is possible to construct a biological molecule, a genome, starting with chemicals in laboratory.11 The second type of Synthetic Biology approach (top-down), has not been considered a threat because it is widely used to create new metabolic pathways in bacteria or yeasts, to produce new compounds such as biofuels, biologically active molecules (often of pharmaceutical value) and chemicals.12,13 Along this way, the synthesis and the insertion of functional biological components into natural genomes is achieved creating metabolic pathways, often combining genes from different organisms. Unfortunately, recently, even this top-down approach raised concerns for the possibility to produce toxic chemicals from microorganisms.14 This type of experiments represents the “Convergence” of Biology towards Chemistry, because chemicals are produced by biological means.15,16 Yeasts are considered excellent cell factories for industrial applications, with the possibility to create engineered cells for efficient production of a desired compound (metabolic engineering). In particular, the yeast Saccharomyces cerevisiae plays an essential role in this field,17 in addition to that of food industry (production of bread, beer, wine and chocolate). With yeasts, it is possible to produce pure amount of chemicals of high quality at low costs and with a standardised process and continuous production all over the year, independent from seasonal harvest.18 Of great interest for the pharmaceutical industry, the biological synthesis could expand the chemical diversity of natural products and their structural complexity;19,20 indeed, model organisms are suitable for advancing new approaches to drug discovery. The most successful production in yeast is the synthesis of the antimalarial artemisin precursor,21,22 Fig.1B, but S. cerevisiae has been used as a biomanufacturing platform for a large number of molecules.23,24 In addition to biofuels (bioethanol and farnesene),25 high value metabolites were produced in yeast:26 penicillin,27 vindoline,28 noscapine,29 anthranilates,30 raspberry ketone,31 resveratrol,32,24 vanillin,33 rose oil and perfumes.34
Illicit Chemicals from Yeast Cells
For the first time, in 2015, illicit molecules (brain acting molecules) were produced applying Synthetic Biology top-down approach to yeast cells. Concerns were raised when the production of codeine and morphine was achieved in S. cerevisiae:35,36 different groups successfully inserted genes from bacteria, opium poppy and sugar beet into yeast, reconstructing the pathway for morphine or codeine production.37,38,39 The entire biosynthetic pathway of thebaine (precursor of morphine) and hydrocodone has been achieved by inserting genes from plants, bacteria and mammals.40 The expression of the entire opiate pathway in a single yeast cell has now become a reality but could be subject to misuse because anyone with access to morphine-producing yeast strains, and with basic fermentation skills, could easily produce morphine in large quantity, starting from glucose.41 Opioids are of great pharmaceutical value because are the primary analgesic alkaloids used for pain relief; the chemical synthesis of these molecules is not commercially competitive, when compared with the extraction from the Papaver somniferum plant; so, with Synthetic Biology tools, it soon will be possible to produce cheaper, less addictive, safer and more effective analgesics. Another type of brain active molecules, which are used to inhibit inflammation and pain, are the cannabinoids, extracted from Cannabis sativa.42 Cannabinoids act on human endocannabinoid system, which is involved in a wide array of bodily processes, including appetite, memory, pain and mood. Cannabis sativa plant makes over 100 different types of cannabinoids, but the most important are tetrahydrocannabinol (THC), which is responsible of psychoactive effects, and cannabidiol (CBD), which has solely therapeutic effects. These molecules bind to different human cannabinoid receptors: THC binds to the cannabinoid CB1 receptor in the brain triggering psychotropic and psychedelic effects, while the cannabidiol binding to the CB2 receptor has an anti-inflammatory effect. The obstacle to the wide use of cannabinoids in medicine is that the extraction of cannabidiol contains a small percentage of THC. The pharmaceutical industry is seeking a synthetic form of cannabis that inhibits inflammation and pain, but without the psychedelic or psychotropic effect. The advantage of using yeast to produce cannabinoids is that strains can be engineered to produce only solely therapeutic cannabinoids, like CBD, and not those that are psychedelic or psychotropic, like THC. In other words, yeast could be used to make pure products used for nutraceutical or pharmaceutical purposes: until now, using yeast cells, Germany’s Technical University of Dortmund, achieved the production of Δ9-tetrahydrocannabinol and cannabidiol; Canada’s Hyasynth Bio produced cannabigerol; England’s Anandia Laboratories make cannabinoids and US BioTork LLC, cannabidiol.
Conclusions on Dual-Use Implication
The pharmaceutical interest on opioids and cannabinoids will result in a further future development of metabolic engineering pathways to improve the yields and produce other molecules acting in the brain. These discoveries could lead to the massive production of illicit drugsin fermenters, with cheap row materials and in an easy way, in any place in the world. Unfortunately, yeast cells, thanks to the harmless nature of this organism, can be easily transported without biosafety restrictions.43 In the future, the bottom-up will meet the top-down technology and it will be possible to introduce into yeast synthetic genome the biological modules containing the entire biosynthetic opiates or cannabinoids pathways and select yeast cells overproducing morphine or cannabidiol. For the above reason, this type of experimentation should rise attention of the scientific community on the so called dual-use technology,44 being this the first case of production of controlled narcotics by synthetic biology. In the light of the fast development of biotechnology, the outreach to academia should be as broad as possible, to capture the attention also of researchers involved in basic science. In the short to medium term, Synthetic Biology is unlikely to pose new risks or threats, but the long-term possibility to insert foreign synthetic genes into a natural organism, with the aim of producing biological weapons or drugs could turn even a harmless organism, such as the Saccharomyces cerevisiae into a dangerous threat. Toxins have already been expressed in microorganisms: bacterial toxins from Escherichia coli, Vibrio cholerae, Bacillus thuringiensis, Clostridium botulinum and Clostridium tetani were successfully expressed in the yeast Pichia pastoris.45 The non-enzymatic heavy chain of the botulinum toxin B was expressed in yeast with the aim of expressing a secretory toxin antigen, and the subunit A (the catalytic subunit) of the ricin toxin has been expressed in S. cerevisiae, to study how ricin kills the cells for the development of a protective ricin antidote.46,47 Finally, engineered anthrax toxin in bacteria was successfully used to selectively deliver toxic molecules in cancer cells, resulting in successful tumor therapy in animal models.48,49,50,51 The examples reported indicate that we are facing a real genomic and biotechnological revolution in life sciences and that researchers who manipulates GRAS organisms should also be aware of bio-threats in the future. Simple actions would be sufficient to prevent the unconscious spread of intangible technologies or strains and plasmids among potential bioterrorists.43
References
- (OPCW)., O. f. (s.d.). Tratto da http://www.opcw.org/fileadmin/OPCW/SAB/en/TWG Scientific_Advsiory_Group_Final_Report.pdf.
- (AG)., A. G. (s.d.). Tratto da http://www.australiagroup.net/en/agm_apr2008.html.
- Schmidt, M. &. Giersch, G. (2011). DNA Synthesis and Security. DNA Microarrays, Synthesis and Synthetic DNA, 285-300.
- van der Bruggen, K. (2012). Possibilities, Intentions and Threats: Dual Use in the Life Sciences Reconsidered. Science and Engineering Ethics, 18(4), 741-756.
- Kelle, A. (2013). Beyond Patchwork Precaution in the Dual-Use Governance of Synthetic Biology. Science and Engineering Ethics, 19(3), 1121-1139.
- Cirigliano, A., Cenciarelli, O., Malizia, A., Bellecci, C., Gaudio, P., Lioj, M., & Rinaldi, T. (2017). Biological Dual-Use Research and Synthetic Biology of Yeast. Science and Engineering Ethics, 23(2), 365-374.
- Lartigue, C., Vashee, S., Algire, M. A., Chuang, R. Y., Benders, G. A., Ma, L., ... & Alperovich, N. (2009). Creating Bacterial Strains from Genomes that Have Been Cloned and Engineered in Yeast. Science, 325(5948), 1693-1696.
- Gibson, D. G., Glass, J. I., Lartigue, C., Noskov, V. N., Chuang, R. Y., Algire, M. A., ... & Venter, J. C. (2010). Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science, 329(5987), 52-56.
- Annaluru, N., Muller, H., Mitchell, L. A., Ramalingam, S., Stracquadanio, G., Richardson, S. M., ... & Linder, M. E. (2014). Total Synthesis of a Functional Designer Eukaryotic Chromosome. Science, 344(6179), 55-58.
- Gibson, D. G., & Venter, J. C. (2014). Synthetic Biology: Construction of an Yeast Chromosome. Nature, 509(7499), 168-169.
- Hutchison, C. A., Chuang, R. Y., Noskov, V. N., Assad-Garcia, N., Deerinck, T. J., Ellisman, M.
a. H., ... & Pelletier, J. F. (2016). Design and Synthesis of a Minimal Bacterial Genome. Science,
b. 351(6280), aad6253. - Cameron, D. E., Bashor, C. J., & Collins, J. J. (2014). A Brief History of Synthetic Biology. Nature Reviews Microbiology, 12(5), 381-390.
- Coumou, H., D Petersen, P., & H Mortensen, U. (2017). Saccharomyces cerevisiae as Cell Factory for the Production of Plant Natural Products. Current Biotechnology, 6(3), 194-204.
- Tucker, J. B. (2010). The Convergence of Biology and Chemistry: Implications for Arms Control Verification. Bulletin of the Atomic Scientists, 66(6), 56-66.
- Schmidt, M., & Pei, L. (2011). Synthetic Toxicology: Where Engineering Meets Biology and Toxicology. Toxicological Sciences, 120(suppl 1), S204-S224.
- Nielsen J, Fussenegger M, Keasling J, Lee SY, Liao JC, Prather K, Palsson B. (2014). Application of Synthetic Biology for Production of Chemicals in Yeast Saccharomyces Cerevisiae. Nat Chem Biol, 10, 319-22.
- Jouhten, P., Boruta, T., Andrejev, S., Pereira, F., Rocha, I., & Patil, K. R. (2016). Yeast Metabolic Chassis Designs for Diverse Biotechnological Products. Scientific Reports, 6.
- Dziggel, C., Schäfer, H. and Wink, M. (2017). Tools of Pathway Reconstruction and Production of Economically Relevant Plant Secondary Metabolites in Recombinant Microorganisms. Biotechnol. J., 12: n/a, 1600145.
- Billingsley, J. M., DeNicola, A. B., & Tang, Y. (2016). Technology Development for Natural Product Biosynthesis in Saccharomyces Cerevisiae. Current Opinion in Biotechnolog., 42, 74-83.
- Smanski, M. J., Zhou, H., Claesen, J., Shen, B., Fischbach, M., & Voigt, C. A. (2016). Synthetic Biology to Access and Expand Nature’s Chemical Diversity. Nature Reviews. Microbiology, 14(3), 135.
- Ro, D. K., Paradise, E. M., Ouellet, M., Fisher, K. J., Newman, K. L., Ndungu, J. M., ... & Keasling, J. D. (2006). Production of the Antimalarial Drug Precursor Artemisinic Acid in Engineered Yeast. Nature, 440, 940-943.
- Paddon, C. J., & Keasling, J. D. (2014). Semi-Synthetic Artemisinin: A Model for the Use of Synthetic Biology in Pharmaceutical Development. Nature Reviews Microbiology, 12(5), 355-367.
- Porro, D., Gasser, B., Fossati, T., Maurer, M., Branduardi, P., Sauer, M., & Mattanovich, D. (2011). Production of Recombinant Proteins and Metabolites in Yeasts. Applied Microbiology and Biotechnology, 89(4), 939-948.
- Li, M., & Borodina, I. (2015). Application of Synthetic Biology for Production of Chemicals in Yeast Saccharomyces Cerevisiae. FEMS Yeast Res, 15(1), 1-12.
- Nielsen, J., Larsson, C., van Maris, A., & Pronk, J. (2013). Metabolic Engineering of Yeast for Production of Fuels and Chemicals. Current Opinion in Biotechnology, 24(3), 398-404.
- Dai, Z., Liu, Y., Guo, J., Huang, L., & Zhang, X. (2015). Yeast Synthetic Biology for High-Value Metabolites. FEMS Yeast Research, 15(1), 1-11.
- Awan, A. R., Blount, B. A., Bell, D. J., Ho, J. C., McKiernan, R. M., & Ellis, T. (2016). Biosynthesis of the Antibiotic Nonribosomal Peptide Penicillin in Baker's Yeast. bioRxiv., 075325.
- Qu, Y., Easson, M. L., Froese, J., Simionescu, R., Hudlicky, T., & De Luca, V. (2015). Completion of the Seven-Step Pathway from Tabersonine to the Anticancer Drug Precursor Vindoline and Its Assembly in Yeast. Proceedings of the National Academy of Sciences, 112(19), 6224-6229.
- Li, Y., & Smolke, C. D. (2016). Engineering Biosynthesis of the Anticancer Alkaloid Noscapine in Yeast. Nature Communications, 7.
- Eudes, A., Benites, V. T., Wang, G., Baidoo, E. E., Lee, T. S., Keasling, J. D., & Loqué, D. (2015). Precursor-Directed Combinatorial Biosynthesis of Cinnamoyl, Dihydrocinnamoyl, and Benzoyl Anthranilates in Saccharomyces Cerevisiae. PloS one, 10(10), e0138972.
- Lee, D., Lloyd, N. D., Pretorius, I. S., & Borneman, A. R. (2016). Heterologous Production of Raspberry Ketone in the Wine Yeast Saccharomyces Cerevisiae via Pathway Engineering and Synthetic Enzyme Fusion. Microbial Cell Factories, 15(1), 49.
- Becker, J.V. et al. (2003). Metabolic Engineering of Saccharomyces Cerevisiae for the Synthesis of the Wine-Related Antioxidant Resveratrol. FEMS Yeast Res, 4, 79-85.
- Brochado, A. R., Matos, C., Møller, B. L., Hansen, J., Mortensen, U. H., & Patil, K. R. (2010). Improved Vanillin Production in Baker's Yeast Through in Silico Design. Microbial Cell Factories, 9(1), 84.
- Gupta, C., Prakash, D., & Gupta, S.A (2015). Biotechnological Approach to Microbial Based Perfumes and Flavours. Journal of Microbiology and Experimentation., 3(1).
- Hawkins K.M. & Smolke C. (2014). Production of Benzylisoquinoline Alkaloids in
a. Saccharomyces Cerevisiae. Nature Chem Bio, 5, 564-573. - Thodey, K., Galanie, S. & Smolke, C. D. (2014). A Microbial Biomanufacturing Platform for Natural and Semisynthetic Opioids. Nature Chem. Biol, 10, 837–844.
- DeLoache, W. C., Russ, Z. N., Narcross, L., Gonzales, A. M., Martin, V. J., & Dueber, J. E. (2015). An Enzyme-Coupled Biosensor Enables (S)-Reticuline Production in Yeast from Glucose. Nature Chemical Biology, 11, 465–471.
- Fossati, E., Narcross, L., Ekins, A., Falgueyret, J. P. & Martin, V. J. J. (2015). Synthesis of Morphinan Alkaloids in Saccharomyces Cerevisiae. PLoS ONE, 10, e0124459.
- Beaudoin, G. A. (2015). Characterization of Oxidative Enzymes Involved in the Biosynthesis of Benzylisoquinoline Alkaloids in Opium Poppy (Papaver somniferum). PhD thesis, Univ. Calgary available at http://hdl.handle.net/11023/2115. In G. A. Beaudoin, PhD thesis (p. available at http://hdl.handle.net/11023/2115.). Univ. Calgary.
- Galanie, S., Thodey, K., Trenchard, I. J., Interrante, M. F., & Smolke, C. D. (2015). Complete Biosynthesis of Opioids in Yeast. 349(6252), 1095-1100.
- Oye, K. A., Lawson, J. C., & Bubela, T. (2015). Drugs: Regulate ‘Home-Brew' Opiates. Nature, 521(7552), 281.
- Zirpel, B., Stehle, F., & Kayser, O. (2015). Production of Δ9-Tetrahydrocannabinolic Acid from Cannabigerolic Acid by Whole Cells of Pichia (Komagataella) Pastoris Expressing Δ9-Tetrahydrocannabinolic Acid Synthase from Cannabis sativa l. Biotechnology Letters, 37(9), 1869-1875.
- Rinaldi, T. (2015). “Poppy” Yeast. EMBO Reports , 16(11), 1410-1410.
- Tucker, J. B. (2012). Innovation, Dual Use, and Security: Managing the Risks of Emerging Biological and Chemical Technologies. MIT Press.
- Gurkan, C., & Ellar, D. J. (2005). Recombinant Production of Bacterial Toxins and Their Derivatives in the Methylotrophic Yeast Pichia Pastoris. Microbial Cell Factories., 4(1), 1.
- Allen, S. C., Byron, A., Lord, J. M., Davey, J., Roberts, L. M., & Ladds, G. (2005). Utilisation of the Budding Yeast Saccharomyces Cerevisiae for the Generation and Isolation of Non‐Lethal Ricin a Chain Variants. Yeast, 22(16), 1287-1297.
- Becker, B., Schnöder, T., & Schmitt, M. J. (2016). Yeast Reporter Assay to Identify Cellular Components of Ricin Toxin A Chain Trafficking. Toxins, 8(12), 366.
- Wein, A. N., Peters, D. E., Valivullah, Z., Hoover, B. J., Tatineni, A., Ma, Q., ... & Liu, S. (2015). An Anthrax Toxin Variant with an Improved Activity in Tumor Targeting. Scientific Reports, 5.
- Liu, S., Liu, J., Ma, Q., Cao, L., Fattah, R. J., Yu, Z., ... & Leppla, S. H. (2016). Solid Tumor Therapy by Selectively Targeting Stromal Endothelial Cells. Proceedings of the National Academy of Sciences., 201600982.
- Chen, K. H., Liu, S., Leysath, C. E., Miller-Randolph, S., Zhang, Y., Fattah, R., ... & Leppla, S. H. (2016). Anthrax Toxin Protective Antigen Variants that Selectively Utilize Either the CMG2 or TEM8 Receptors for Cellular Uptake and Tumor Targeting. Journal of Biological Chemistry., 291(42), 22021-22029.
- Bachran, C., & Leppla, S. H. (2016). Tumor Targeting and Drug Delivery by Anthrax Toxin. Toxins., 8(7), 197.